| Pages:
1
2 |
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Well what do you have?
There are hundreds of ways to do it but depends what you got on hand and your jerry rigging skills.
I've made gas tight seals with toilet paper, Teflon tape and some capillary tube or needles.
So what do ya got.
[Edited on 21-3-2017 by XeonTheMGPony]
|
|
|
Db33
Hazard to Others
  
Posts: 206
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
i have a lot of flasks, 3 neck and 1 neck and some 2 neck. Round bottom and flat bottom. I plan on getting a nitrogen tank from Amazon. I also have
access to various size syringes and needles if that would help, also balloons, etc...
Xeon if you can give me an example of some ways to jerry rig it id appreciate it
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Ok get a nice medium bore needle, now tightly roll up some toilet paper around the needle till it is a tight fit into the flask neck, now take some
Teflon tape and care fully expand it and wrap it care fully around the tissue paper till it is an even thick coat.
this will hold up plenty well for 1 to 2 runs, I used this to seal a nitric acid distillations! You can make it more permanent by soaking superglue
into the tissue paper.
Opt 2 is coating a marshmallow care fully in Teflon tape as well works then driving the needle through, use some copper wire to hold it in tight for
both options.
[Edited on 23-3-2017 by XeonTheMGPony]
|
|
|
Booze
Hazard to Others
  
Posts: 121
Registered: 26-2-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Db33  | | its not a question of what gas to use, im just wondering how to get it from the tank into the flask and keep it there. |
All you need to do is get the gas in and stopper it off. The rubber stopper should keep gasses from getting in pretty good.
Also, this may be a dumb question, but why do you need a nitrogen atmosphere when our atmosphere is already 70% nitrogen? Is the reaction oxygen
sensitive?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2789
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I think maybe a solution of copper and ascorbic acid can be used as an oxygen scrubber. Subject to degradation of ascorbate of course. Ascorbate is
cheap enough that you could deoxygenate a few dozen liters of air by this method -- you just need some means to use it.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
My idea for a cheap and easy inert atmosphere: Get two five-gallon (20-liter) buckets, with at least one airtight lid. Fill one with water, then put
your favorite oxygen/CO2 scavengers in the other. I recommend Ca(OH)2 in water for CO2, and those Hot Hands reduced-iron hand warmers, torn open, for
oxygen. Now rig the lid of the bucket up so that you can have a tube coming in, that goes nearly to the bottom, and a tube going out of the top, that
doesn't dangle inside the bucket at all. Check to make sure that the volume of the water plus your scavengers won't reach the outlet of the bucket.
After leaving it overnight with the lid on, you can then just put the bucket of water above the bucket with the scavengers in it, and siphon water to
it as you need inert gas. Use a drying tube, since there will obviously be moisture in the air, and an on/off valve to turn the inert gas on and off.
The siphon will stop moving water when the pressure inside the second bucket builds up enough to counter it. Seems an easy enough to get a decent
amount of inert gas.
As a bonus, you have a bucket of water in the end that you can throw a vessel into if it's releasing cyanide gas or acidic vapors unexpectedly, and
have those vapors be readily neutralized.
|
|
|
Db33
Hazard to Others
  
Posts: 206
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
So if i was doing a 24 hour reflux... lets say in a 3 neck round bottom flask. If i stoppered 1 neck, then using a hose just flushed the entire flask
with nitrogen for like 60 seconds and then stoppered the 2 side necks and had a reflux condenser on the top with some a drying tube on top of that.
would that be able to keep most of the nitrogen in and the air out?
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I think usually they would use a bubbler instead of a drying tube. It would be filled with mineral oil, sulfuric acid, or some other oil that has
desirable properties and preferably won't react violently if it somehow mixes with your reaction mixture.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
you need a balloon to act as a pressure buffer int hat instance, another method I fancy is to make a saturated solvent atmosphere, so what ever the
most volatile part of the reaction is add a bit more Ml then pull a deep vacuum while warming and no cooling, once it starts to off gas at the top of
the reflux condenser put a stopper in and turn on cooling.
This causes a deep vacuum and the only thing in it is your solvent, Naturally this depends on good sealing! and it significantly reduces the boiling
temp, so if that is integral to the reaction that method won't work.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
One of these could serve as a bubbler. You'd put just enough oil in it to prevent air from getting into the apparatus while still allowing gases from
the reaction to escape. If you have a nitrogen tank, you could let gas slowly diffuse through the apparatus and exit by bubbling through the oil (I
think this is called running a reaction in a "stream of nitrogen").
I don't know what your reaction is, but the balloon idea might be a good one. A little positive pressure in the apparatus would help to keep out
unwanted gases and vapors. Of course, you want to make sure that your balloon doesn't get eaten up by corrosive vapors or solvents, and if the
reaction produces large amounts of gases, a balloon might not be able to contain them.
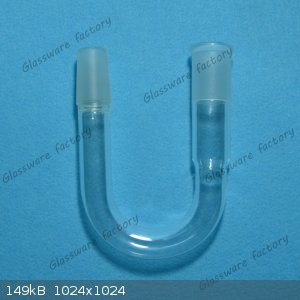
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
JJay has an excellent point! exactly what IS the reaction, we're trying to hit the toilet in the dark here and that tends to make a mess of it!
More details the more accurate the solution can be offered.
|
|
|
Db33
Hazard to Others
  
Posts: 206
Registered: 25-11-2016
Member Is Offline
Mood: No Mood
|
|
the reason the nitrogen atmosphere is needed is because the reducing agent is sodium triacetoxyborohydride, which is sensitive to water and air.
|
|
|
Chemetix
Hazard to Others
  
Posts: 375
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
There is the Schlenk manifold option, a flask with a tap on it to evacuate and fill with N2 is the standard accessory. Jerry rigging the concept is
doable for a back yarder I'd guess.
That's what they look like when set up.

[Edited on 27-3-2017 by Chemetix]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
I looked into getting Nitrogen, although in the end for me Hydrogen turned out to better.
Where I live Nitrogen didnt seem that easy or particularly cheap to get hold of, also I didnt need much. But I did find a source in the end that is
probally availiable in more places than you think.
I noticed a tyre and MOT place had a sign saying, "Nitrogen tyre filling service". Now I have NO IDEA why Nitrogen is sometimes used in car tyres, but
apparently it is.
So in my case I used a old small tractor, front wheel inner tube, I took it along to the tyre place and they filled up the inner tube with nitrogen
for me.
Actually the guy was really cool and didnt charge me for it. Anyway in a push it might be a solution for you, I am told tyre specialist often have
nitrogen to fill car tyres with.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Lol it is essentially a scam unless you're a formula 1 racer, Nitrogen expands less under the influence of temperature so the tires remain at a more
constant pressure.
But it sounds exotic to your average sheeple consumer so an easy extra buck for the companies pimping it out. But good score on a way to make some
real use out of that scam.
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by XeonTheMGPony  | Lol it is essentially a scam unless you're a formula 1 racer, Nitrogen expands less under the influence of temperature so the tires remain at a more
constant pressure.
But it sounds exotic to your average sheeple consumer so an easy extra buck for the companies pimping it out. But good score on a way to make some
real use out of that scam. |
Thanks for that! I was going to get my mum to fill her tyres with it! All the guy told me was "its used for low profile, high performance tyres".
Sounded kind of neat......
But yes I still have most of my nitrogen in the inner tube  . Although now I use
hydrogen for my methane stuff. . Although now I use
hydrogen for my methane stuff.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Air is predominately Nitrogen! even low profile tires there is zero need for it as airs expansion is not that drastic with tires.
It is just a hilarious cash grab! Just make sure the tires have good tread and are at proper pressure and you'll be fine! and all ways fill when cold!
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by XeonTheMGPony  | Air is predominately Nitrogen! even low profile tires there is zero need for it as airs expansion is not that drastic with tires.
It is just a hilarious cash grab! Just make sure the tires have good tread and are at proper pressure and you'll be fine! and all ways fill when cold!
|
Funny how with most things, the more you take out, the more it costs  . I am on
alot of Gluten free stuff, it costs a fortune! Same with Nitrogen, take a few other gases out of air and charge a fortune for it . I am on
alot of Gluten free stuff, it costs a fortune! Same with Nitrogen, take a few other gases out of air and charge a fortune for it  . .
I must go back and take a picture of the sign, it will make you laugh! The claims are many, everything from more MPG, to shorter braking distance  . .
Then again the charge £2 for normal air to inflate your tyres in the garage from a machine!! If nothing else, it might help someone out with a source
for small amounts and a way to transport it.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Oh we have the same garbage here, I nearly soiled my self laughing when the guy said I "should" use nitrogen for my electric car, I said ya but then
I'll have to charge with lighter electricity to see any real change!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Quote: Originally posted by NEMO-Chemistry  | Funny how with most things, the more you take out, the more it costs  . I am on
alot of Gluten free stuff, it costs a fortune! Same with Nitrogen, take a few other gases out of air and charge a fortune for it . I am on
alot of Gluten free stuff, it costs a fortune! Same with Nitrogen, take a few other gases out of air and charge a fortune for it  . .
I must go back and take a picture of the sign, it will make you laugh! The claims are many, everything from more MPG, to shorter braking distance  . .
Then again the charge £2 for normal air to inflate your tyres in the garage from a machine!! If nothing else, it might help someone out with a source
for small amounts and a way to transport it. |
Technically, what they're saying is accurate. Nitrogen is slightly polar, and won't diffuse through a tire as much as oxygen does. Also, a car tire
that got hot enough could theoretically pyrolyze more readily if there was oxygen diffusing through it. I mean, filling your tires with nitrogen WILL
lead to an increase in all the things they mentioned; what they don't tell you is that increases will likely be on the order of 0.01%, which is hardly
something that most people would notice.
The cost of stuff is usually based on how much processing has to go into it, as well as how much they can sell. Putting stuff into something is easy;
taking stuff out is much harder. Any chemist who as accidentally used the wrong stir rod when doing multiple reactions at once has probably learned
that the hard way.
[Edited on 3/31/17 by Melgar]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by XeonTheMGPony  | | Oh we have the same garbage here, I nearly soiled my self laughing when the guy said I "should" use nitrogen for my electric car, I said ya but then
I'll have to charge with lighter electricity to see any real change! |
Where do you buy your lighter electric from? I have a heavy battery for the boat, lighter electric would make moving it easier  . .
Is it expensive? Couldnt find any on ebay, so I guess its banned in the UK. Maybe its some kind of precursor? 
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
I lived off grid so I know all about heavy awkward So lets start selling helium refills for batteries! lol batteries, I just kept telling the supplier
they needed to hurry up and make lead lighter too!
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Barely, if at all
https://en.wikipedia.org/wiki/Charles%27s_law
(and changes in the elasticity of the rubber would make a lot more difference.)
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Melgar  |
Technically, what they're saying is accurate. Nitrogen is slightly polar,
[Edited on 3/31/17 by Melgar] |
That's a clever trick for a diatomic molecule.
Hypothetically, the fact that nitrogen has more "minor" isotopes may make it true, but I'd not want to have to measure the dipole moment of the
15N14N molecule
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Hence why it is a SCAM or did you miss that part? but people who buy into it think that's the reason to use Nitrogen, it is magical after all.
In refrigeration testing it has less pressure drift as it is OFN dry, so the pressure swing Vs temp is easily accounted for, but for a tire it is
meaningless.
|
|
|
| Pages:
1
2 |