manzil_zaheer
Hazard to Self
 
Posts: 62
Registered: 2-6-2006
Location: India
Member Is Offline
Mood: Good
|
|
Ethanoic Acid
What is the oxidation state of carbon in ethanoic acid or acetic acid?
is one carbon +2, and other -2 or one carbon +3 and the other -3 or one carbon +4 and other carbon -4.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
-3,+3.....
http://en.wikipedia.org/wiki/Oxidation_states#Calculation_of...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
I have to disagree with that.
Oxidation number is determined by considering what the charge would be on an atom if the electrons in each covalent bond were assigned as belonging
purely to the more electronegative atom. In the case of acetic acid, the two carbons are NOT equivalent, as they have different atoms attached to
them, and as a result will have different electronegativity.
The carbon in the COOH group is joined to two oxygens, which are electron withdrawing, making that carbon delta +. As a result, this carbon will be
more electronegative (as the partial positive charge means that it will attract electrons more strongly), so the electrons in the C-C bond will NOT be
equally shared. The C-C bond will be polarised such that the electrons will be on average closer to the carbon of the COOH group than the carbon of
the CH3 group. So the carbon on the COOH bond will have lost control of 3 electrons (from the C-O and C=O bonds), but gained control of one electron
(from the C-C bond), giving it an oxidation number of +2. The carbon in the CH3 group will have gained control of 3 electrons (from the C-H bonds) and
lost control of one (from the C-C bond), giving it an oxidation number of -2.
[Edited on 18-11-2006 by Pyrovus]
[Edited on 18-11-2006 by Pyrovus]
Never accept that which can be changed.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The ambiguous concept of "oxidation number" is purely formal and is calculated like you described with the difference that due to its formal
definition you can not extend it to the concept of electron density on carbon atoms as you imply. For some reasons organic chemists adapted the
concept of oxidation number in a way that only the first attached atom is considered in the C-C bond. It does not matter if the neighboring C is part
of COOH or CH3, it is still considered as zero contributing regarding the oxidation number. I guess it was a matter of simplification else things
would become really complicated and the oxidation number would not be an integer anymore  . .
And thus Solo gave the correct numbers. It is however true that in heteroatoms the valency still needs to be considered even in organic compounds. For
example hydride/hydrogen, amino/nitro, thio/sulfoxyde etc.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
With the admitted exception of the wikipedia article, all the places I've seen oxidation number defined purely in terms of the electronegativity
of the two atoms involved in a bond. And while your periodic table might give carbon an electronegativity of 2.55, this only applies to the
elemental form. Once you start adding or removing electrons to an element, you alter it's electronegativity - after all, electronegativity is a
measure of an atom's ability to attract electrons. Putting a positive charge on an atom will increase it's ability to attract electrons, for instance,
so it makes sense that it's electronegativity will increase in this situation. As a result of this, it is very possible for atoms of the same element
to have different electronegativities depending on their chemical environment.
As far as chemical behaviour is concerned, considering the difference in electronegativities between atoms of the same element caused by differing
chemical environments gives a more accurate picture of how that atom can be expected to behave.
For instance, the complex of Cl2 with AlCl3, formed as an intermediate in the catalytic chlorination of aromatic compounds, has the following
structure:
Cl - Cl(+) - Al(-)Cl3
The chemical behaviour of the chlorine on the left is consistent with chlorine in the +1 oxidation state, not the 0. The whole reason
why AlCl3 is used to catalyse chlorinations is because it is able to make the chlorine on the left behave as
Cl+, which is more electrophilic than plain Cl2. This can be explained by the fact that the chlorine it is joined to has a formal positive charge,
thereby increasing it's electronegativity, and causing the electron pair shared by both atoms to be closer to the positively charged chlorine.
It certainly seems absurd that a chlorine atom clearly behaving as if it were in the +1 state should be classed as being in the 0 state, anyway.
[Edited on 25-11-2006 by Pyrovus]
Never accept that which can be changed.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
FYI...
"Its" = posessive
"It's" = contraction of "it is"
You get off with a warning,
Deputy S.E.D. Apostrophe Police
Tim
[Edited on 11-26-2006 by 12AX7]
|
|
|
manzil_zaheer
Hazard to Self
 
Posts: 62
Registered: 2-6-2006
Location: India
Member Is Offline
Mood: Good
|
|
Ok so whose reasoning is most appropriate?
What should i think about it?
|
|
|
manzil_zaheer
Hazard to Self
 
Posts: 62
Registered: 2-6-2006
Location: India
Member Is Offline
Mood: Good
|
|
Why no one is replying??
Please tell which reasoning is most appropriate. I am also confused in this particular case.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Hello,
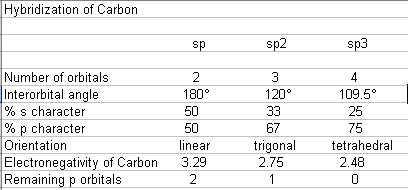
I have always considered the bonding of carbon atoms as a matter of orbital hybridization rather than "oxidation state or valence". Carbon has one 2s
and three 2p orbitals. The electron distribution in these orbitals (orbital overlap) determines the bonding order of the carbon. A nice table
connecting this to electronegativity is given by:
Scudder, Paul H. (1992) Electron Flow in Organic Chemistry. John Wiley and Sons Inc. QD251.2.S39, ISBN 0-471-61381-9. pp. 13.
(which is an excellent and inexpensive softcover book).
-->this is attached as a picture, the editor just mushes the table together.
To clarify, sp or sp1=triple bond, sp2=double bond and sp3 single bond.
C=O counts as an sp2 hybridized carbon.
Also, please look up Lewis structures and resonance forms; this helps out with the above significantly (and makes sure you don't break your octets).
Good luck!
O3
[Edited on 13-12-2006 by Ozone]
[Edited on 13-12-2006 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|