bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Secondary amine mannich condensation + ring formation.
If a mannich condensation was carried out between dimethylamine, formaldehyde, and methyl ethyl ketone would the ring close? I assume no, how far am i
off base here? Maybe with methyl ethyl ketene the alkene would allow the ring to close with a ruthenium catalyst?
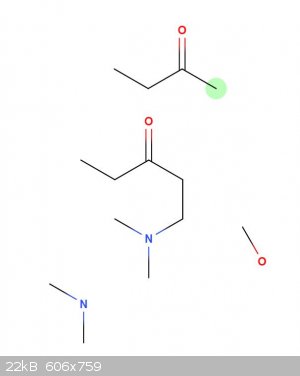
This is not homework, just my personal question. thanks for the help.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bmays  | | If a mannich condensation was carried out between dimethylamine, formaldehyde, and methyl ethyl ketone would the ring close? I assume no, how far am i
off base here? |
your first step is itself wrong since the addition will happen to the more substituted carbon.Secondly,since formaldehyde is a nifty and highly
reactive molecule,the iminium cation formed will keep attacking the carbons till there is no alpha H left to play with.I don't think it will be
interested to form rings.But you can go through this paper-http://link.springer.com/article/10.1007/BF01151327
| Quote: | | Maybe with methyl ethyl ketene the alkene would allow the ring to close with a ruthenium catalyst? |
won't the ketene react with the amine to form an amide ?
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
There are many well documented ring forming reactions to give piperidones using the Mannich reaction in natural product syntheses. However, they all
employ primary amines and are usually run under carefully controlled conditions.
AvB
|
|
|
Cryolite.
Hazard to Others
  
Posts: 269
Registered: 28-6-2016
Location: CA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AvBaeyer  | There are many well documented ring forming reactions to give piperidones using the Mannich reaction in natural product syntheses. However, they all
employ primary amines and are usually run under carefully controlled conditions.
AvB |
There are? All I found in a brief literature search I did a little while back was a reference to the synthesis of bispidinones. (effectively the
product of two further Mannich additions to the piperidone ring itself) They looked like interesting ligands for transition metals, but I couldn't
find anything else interesting to do with them, nor could I find documentation of true piperidone synthesis.
[Edited on 25-10-2016 by Cryolite.]
|
|
|
bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
CuReUS you're right it would have been 5th not 4th substitution. Yes another oversight ketenes are highly reactive and would react with the amine
immediately.
http://www.sciencemadness.org/library/books/organic_reaction...
On page 305
| Quote: | | Frequently such products, derived from two molecules of ketone, two molecules of formaldehyde, and one molecule of primary amine, are unstable and
readily undergo cyclization. The compounds obtained from acetone, formaldehyde, and methylamine are illustrative. The product to be expected from a
Mannich reaction involving an ammonium salt is a primary amine. In many cases, the primary amine so produced reacts further, as above, to form a
secondary amine, a tertiary amine, or a cyclic substance. The situation is further complicated by the fact that methylamine, produced from the
ammonium salt and formaldehyde, also takes part in the reaction. For example, the compounds shown above as products of acetone, formaldehyde, and
methylamine hydrochloride are also obtained from acetone, formaldehyde, and ammonium chloride. |
Page 313
| Quote: | | When a primary amine is used with a polycarbonyl compound which contains reactive hydrogen atoms on carbon atoms located in the 1,3-positions with
respect to each other, then cyclic products may be expected. Thus, esters of a,a-diethylacetonedicarboxylic acid react with formaldehyde and
methylamine to give pyridones. If the pyridone contains hydrogen atoms on the 3- and 5-carbon atoms, the condensation may be carried one step further
and a bicyclic system may be produced. For example, the pyridone obtained by a reaction of the Mannich type from methyl acetonedicarboxylate,
acetaldehyde, and methylamine can be condensed with formaldehyde and methylamine. The name "bispidin" has been suggested for the bicyclic ring system
produced in such reactions |
There is a chart on page 331 which may also be of interest. No 4 substituted piperidone synthesis, closest thing might be the n-methyl derivative.
Methylamine, formaldehyde, acetonedicarboxilate diethylester. Look on the chart the yield with acetone is 0.
I have been experimenting with this reaction and often get polymers like goop or oils. Bispidin's or trispidi's maybe.
[Edited on 27-10-2016 by bmays]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bmays  | | products derived from two molecules of ketone, two molecules of formaldehyde, and one molecule of primary amine, are
unstable and readily undergo cyclization. |
the key word here is two.It won't be an intramolecular cyclisation using only one ketone like you want
| Quote: | | When a primary amine is used with a polycarbonyl compound which contains reactive hydrogen atoms on carbon atoms located in the 1,3-positions with
respect to each other, then cyclic products may be expected. Thus, esters of a,a-diethylacetonedicarboxylic acid |
comparing acetone dicarboxylic acid with MEK is wrong because the former molecule is symmetric and has additional COOEt molecules driving the
reaction.
to get what you want,I think you should use methyl vinyl ketone instead of MEK.Also,don't use secondary amines,stick to primary amines or ammonia
[Edited on 27-10-2016 by CuReUS]
|
|
|
bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
True. Yes i've read the acidity of the 1, 3 poistions of acetonedicarboxic acid helps speed up the reaction. As it would with methyl vinyl ketone i
assume. I'm interested in an unsubstituted ring though.
[Edited on 30-10-2016 by bmays]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
you will get an unsubstituted ring with MVK also  .To avoid further
confusion,please tell exactly what you want to synthesise and give the CAS no of the compound,if possible .To avoid further
confusion,please tell exactly what you want to synthesise and give the CAS no of the compound,if possible
[Edited on 30-10-2016 by CuReUS]
|
|
|
bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Cas: 41661-47-6
Sorry my first post probably generated the confusion it was poorly thought out. I was looking for a way around ring formation via the mannich because
it always forms tar. That's why the Petrenko-Kritschenko reaction uses benzaldehyde i guess.
So you're saying with MVK, one mole of formaldehyde, and one mole of a primary amine the resultant molecule will form a ring at the alkene and the
nitrogen
**edit: scratch this, you said the more substituted alpha h position will be attacked first**
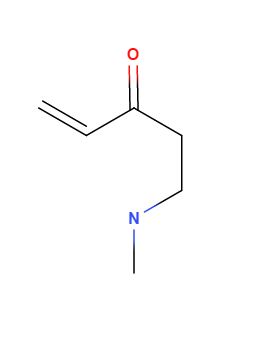
I must be off base here. This isn't a mannich is it? I'm confused, you said two moles of ketone? Wouldn't the classic ring forming mannich be 1mol
mvk, 2 mol formalin, 1 mol methylamine? and won't this be the result? Or maybe it will be lost somehow.
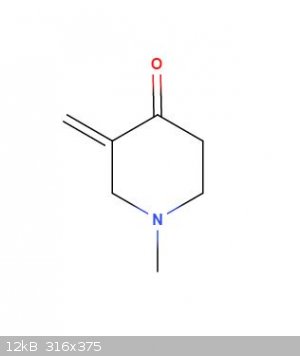
thanks for the help
[Edited on 30-10-2016 by bmays]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bmays  | | So you're saying with MVK, one mole of formaldehyde, and one mole of a primary amine the resultant molecule will form a ring at the alkene and the
nitrogen |
yes. It's called the aza- michael reaction http://www.masterorganicchemistry.com/reaction-guide/14-addi...
in our case,instead of an enolate,the N atom acts as the nucleophile and completes the ring.
the idea which I suggested earlier used both the mannich as well as the aza-michael reaction.The beauty of the reaction was that it didn't matter
whether the mannich reaction(with the methyl group of MVK,amine,HCHO) happened first follwed by the aza- michael reaction(with the double bond of MVK
and the amine) or vice-versa because both the pathways would give 4-methylpiperidone in the end.But since you want piperidone only,my route is no
longer useful.There is also no use talking about primary or secondary amines,ammonia it will have to be.So DVK is the way to go.Sadly,both the papers
you linked are not of much use.The russian paper is not available online(they have papers starting from 2001) and the japanese paper gives the
synthesis of MVK
http://www.journal.csj.jp/doi/pdf/10.1246/cl.1988.1991
the best thing to do would be to buy some DVK and react it with NH3 to see if the reaction works.Then we can worry about synthesising
DVK.See this paper where they synthesise triacetone amine from phorone and NH4OH http://www.tandfonline.com/doi/full/10.1080/1525777070149052...
| Quote: | | I'm confused at the two moles of ketone bit |
There is nothing to be confused about.You want piperidone.You won't get that using the "2 moles of ketone" method.You would get this and its isomer.Their structures are given on pg 305 of the book you linked above.
[Edited on 30-10-2016 by CuReUS]
|
|
|
bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Ahh i thought it looked like a michael addition, azide to methylene. Indeed quite a nice way of building a piperidone. The methyl won't be a problem,
either i will modify my plans downstream or i have some boron tribromide in my freezer which needs some use before the bottle i put it in degrades and
lets loose an epic Halloween smoke show. However i think DVK might be my first experiments simply because the LD50 of MVK is a bit scary, although i
guess all unsaturated vinyl ketones will be toxic.
Thanks, i couldn't find that paper. I should have figured it would take a little more then 20% KOH to make a reaction between formaldehyde and acetone
happen and 4% yields aren't very good haha. I wonder if the Russians had a better way only DVK was listed as the product whereas the Japanese paper
listed MVK and DVK as products.
I've got some reading and maybe some experiments to do now, thanks for the help.
[Edited on 30-10-2016 by bmays]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
are you sure BBr3 is used for N-demethylation ? https://en.wikipedia.org/wiki/Demethylation#N-Demethylation
if you have decided to use DVK,don't forget to go through the triacetoneamine paper I linked above.Phorone is quite similar to DVK.
|
|
|
bmays
Hazard to Self
 
Posts: 55
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Right. Never done an N-demethylation, cyanogen bromide sounds interesting and not too hard to prepare surely easier then BBr3 or anhydrous AlCl3.
Interesting the melting point and boiling point of BrCN are so close together. I see phorone would give the 2,2,6,6-Tetramethyl, i don't have access
to that article though. Maybe at some future time.
|
|
|