DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Molten potassium thiocyanate
I recently noticed that potassium thiocyanate has a very low melting point when anhydrous; 173.2C. I did some googling and couldn't come up with much,
so I wanted to ask here if electrolysis of molten potassium thiocyanate or perhaps sodium thiocyanate (MP 287C, but decomposes at 307C) could perhaps
result in the formation of the alkali metal.
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
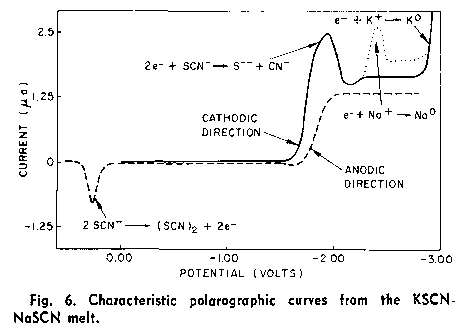
I also did some googling and in 2 minutes (changing keywords) found this: "Advances in Molten Salt Chemistry vol.3" and from it: R. E. Panzer and
M. J. Schaer, J. Electrochem. Soc. 112:1136 (1965).
Shortly: Na(K) reacts with SCN(-) forming S(2-) and CN(-) ions.
In another words, reaction:
Na(K)(+) +e → Na(K)
occurs at more negative potential than reaction:
SCN(-) + 2e → S(2-) + CN(-).
The picture explains everything.
Слава Україні !
Героям слава !
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
So, it's not possible, or it is possible just under certain conditions?
|
|
|