chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
dimethylation procedures & reagent choice
I want to dimethylate an amine. The compound is an aromatic ethyl amine. The Ar group is probably irrelevant; call it pyridine, phenyl or indole..etc.
The choices of methylating agents are dimethyl sulfate or methyliodide (I have both). I don't have methyltriflate. (Me)2I is less toxic than (CH3)2SO4
and the procedure I have a write up for. The write up indicates formation of the quaternary amine and subsequent demethylation to the dimethylamine.
Would the use of dimethylSO4 lead to the quaternary salt or could use of 2eq. of this agent get me the dimethylamine with less hassle? i.e. without
the demethylation step. Another route I read is altogether different using formaldehyde (2eq.) and NaCNBH3. I don't understand the mechanism for this
last and would appreciate a relevant read!! I'm leaning toward the formaldehyde procedure but before I attempt it I need to understand how it works
(mech). Also considering dimethyl carbonate. Could I convert one of the two I have to the carbonate? Surely but how to proceed?
[Edited on 18-4-2016 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Dr.Q
Harmless

Posts: 24
Registered: 18-11-2012
Member Is Offline
Mood: No Mood
|
|
Methylating the amine with sn2 reaction ( in this case methylating with MeI or
Me2SO4) is not very usefull because no matter how much you add equivalent amounts you will always get a mixture of primary secondary tertiary and
quaternary amines. When an amine is methylated its nuclephility is increases also and thus it gets more reactive. The reaction does not go step by
step methylation .
I think using reductive amination reaction with NaCNBH3 or NaBH(AcO)3 is your best bet . Easy controllable and higher yields.
Neverthless , if you want do methylation with sn2 reaction , then the best route would be , first methylating the amine to quaternary salt and then
demethylating it with etanolamine .
OR
methylating it in the pressence of Hünicks Base . So the reaction will stop at tertiary amine formation and no quarternary amine .
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
it is relevant if its an indole because then you would also have to think about ring N methylation.
Its obvious that you have DMT in mind,so why don't you say it upfront ?
Quote: Originally posted by Myeou  |
I think using reductive amination reaction with NaCNBH3 or NaBH(AcO)3 is your best bet . Easy controllable and higher yields. |
what is the need to use hydrides when the reduction can be done using HCOOH itself ? 
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
So long as competing reactions won't occur (e.g. Pictet-Spengler), I'd recommend looking into using formaldehyde/formic acid (Eschweiler-Clarke
reaction). No risk of forming quat salts, and cheaper/less hazardous than typical methylating agents.
EDIT:
Quote: Originally posted by CuReUS  |
it is relevant if its an indole because then you would also have to think about ring N methylation.
Its obvious that you have DMT in mind,so why don't you say it upfront ?
|
Indole-N-alkylation typically requires deprotonation with LDA/NaH and alkylation with alkyl halide. I suspect an aliphatic amino group could be
methylated in preference to the indole without too much difficulty. The regiochemistry of the substrate may also affect the outcome of the reaction in
some cases.
[Edited on 18-4-2016 by DJF90]
|
|
|
Dr.Q
Harmless

Posts: 24
Registered: 18-11-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
it is relevant if its an indole because then you would also have to think about ring N methylation.
Its obvious that you have DMT in mind,so why don't you say it upfront ?
Quote: Originally posted by Myeou  |
I think using reductive amination reaction with NaCNBH3 or NaBH(AcO)3 is your best bet . Easy controllable and higher yields. |
what is the need to use hydrides when the reduction can be done using HCOOH itself ?  |
becaus eof low yields and it would be hard to seperate and also for DMT an acidic condition could be cause to from cylyzation from the nucleophilic
attack of nitrogen in the indole ring to the imin.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Chemrox,
At the weekend I came across a few di- and mono-methylating processes while I was searching for N-methyl-aniline and N,N-dimethylaniline. I know it is
not the same because it is an aromatic amine but maybe worth checking formic acid/formaline or trimethylphosphate or hexamine or dimethyl-oxalate as
methylating agents in general. You may find something worthwhile. :-)
For example there is an interesting method which utilizes alkyl-, benzylic-, or allyl-halides as alkylating agents in the presence of
NaHCO3 and reportedly has good yields without the formation of quaternary products. The article also has a nice overview of
amine-alkylation methods in general:
Aqueous-mediated N-alkylation of amines
DOI:10.1002/ejoc.200600937
As for the reductive methylation this article may interest you:
Reductive methylation of primary and secondary amines and amino acids by aqueous formaldehyde and zinc
Tetrahedron Letters 48 (2007) 7680-7682
|
|
|
Nicodem
|
Thread Moved
18-4-2016 at 08:48 |
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Pummukli-any chance you could post those refs in refs?
I found the attached. I don't understand the dissolving metal reduction step in this example. Also while the Eschweiler-Clarke reaction seems on
point, why are the examples I read using NaCNBH3 instead of formic/formaldehyde? Is the latter too slow or too harsh?
Also wondering whether STAB would be an alternative to NaCNBH3? Both use acetic acid and I have both hydrides. But really baffled by the dissolving
metal.
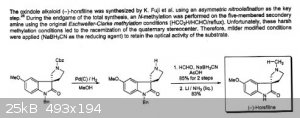
demethylation using ethanolamine? I'm very interested.. can you provide refs for this?
[Edited on 18-4-2016 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
It cleaves the N-Bn bond. The mechanism is similar to the OH-Bn bond cleavage in pseudoephedrine reductions using lithium in ammonia.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Thanks-that is consistent with the diagram too..
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Aqueous-Mediated N-Alkylation of Amines
Chingakham B. Singh,Veerababurao Kavala
Eur. J. Org. Chem.
2007, 1369–1377
DOI: 10.1002/ejoc.200600937
Abstract
Direct N-alkylation of primary amines to secondary/tertiary
amines and of secondary amines to tertiary amines has been
achieved in excellent yields by employing alkyl, benzylic
and allylic halides in the presence of NaHCO3 in an aqueous
medium at an elevated temperature. Amines of different
stereoelectronic nature react with ease with different halides.
The selective formation of secondary amines and the formation
of three different substituted tertiary amines are some of the interesting features of this methodology. Reaction in an
aqueous medium, operationally convenient conditions, excellent
yields and innocuous byproducts, and the absence of
transition-metal catalysts, expensive bases, solid supports
and the formation of undesired quaternary ammonium salts
makes this method a green chemical process.
--------------------------------
Reductive methylation of primary and secondary amines and
amino acids by aqueous formaldehyde and zinc
Renato A. da Silva,a Ida´lia H. S. Estevam
Tetrahedron Letters
48 (2007) 7680–7682
doi:10.1016/j.tetlet.2007.08.092
Abstract
Amines can be methylated when treated with formaldehyde and zinc in aqueous medium. Selective mono- or dimethylation
can be achieved by proper choice of pH, stoichiometry and reaction time. This method can also be applied for amino acids.
Attachment: Aqueous-Mediated N-Alkylation of Amines.pdf (196kB)
This file has been downloaded 746 times
Attachment: Reductive methylation of primary and secondary amines and amino acids by aqueous formaldehyde and zinc.pdf (98kB)
This file has been downloaded 538 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I just found out why HCOOH cannot be used.I was not aware of this before 
| Quote: | | Note by Rhodium: The Pictet-Spengler side-reaction occurs whenever a reductive amination of Tryptamine is performed under acidic conditions (such as
HCOOH/HCHO methylation) |
from https://www.erowid.org/archive/rhodium/chemistry/tryptamine2...
I apologise for my stupidity 
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Chemrox, the following two articles contain experimental information you may find useful. They both use sodium borohydride in water to reductively
alkylate tryptamine derivatives with excess formaldehyde, using methanol as the solvent. I imagine this would probably also be a decent general
method for methylating primary amines.
The two files that look like they have the same name are the article and its supplementary materials (containing experimental procedures),
respectively.
Attachment: Synthesis of 5-(sulfamoylmethyl)indoles.pdf (152kB)
This file has been downloaded 693 times
Attachment: A novel and convenient route for the construction of 5-((1H-1,2,4-triazol-1-yl)methyl)-1H-indoles and its application in (607kB)
This file has been downloaded 604 times
Attachment: A novel and convenient route for the construction of 5-((1H-1,2,4-triazol-1-yl)methyl)-1H-indoles and its application in (2.8MB)
This file has been downloaded 585 times
[Edited on 19-4-2016 by Crowfjord]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
what about using Hunig's base and MeI?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
It looks like I could N-alkylate trytpamine with Ch3I in acetonitrile with 1.5 eq of Hunig's base
Attachment: EJ-1549CP.Hunig's base.pdf (54kB)
This file has been downloaded 553 times
[Edited on 20-4-2016 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|