Farenimio
Harmless

Posts: 4
Registered: 12-2-2016
Member Is Offline
Mood: No Mood
|
|
what can be done with Diclofenac?
I have access to a good amount of diclofenac but i am not very sure what can be done with it... what reactions do you think that can be done with
diclofenac? any suggestion will be well received
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2789
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You could try making the nitrosamine; it's probably colorful.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
And carcinogenic too, most likely . . . ?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2736
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Try making the ester like AvBayer did for ibuprofen. Might be harder due to the amine in it. Or try reacting the amine with acids or alkylating
it. It also works well for arthritis and general pains, that would be my use, as I seem to get more of them nowadays.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You can cylize it to the corresponding oxindole. This should be doable by refluxing in acetic acid with a few mol% of some sulfonic acid.
Alternatively, maybe refluxing in xylenes would be enough, especially in the presence of a few mol% TsOH or boric acid and a Dean-Stark trap. See
literature examples.

You can use the oxindole in further reactions. Its methylene group is moderately activated and can be condensed with properly reactive carbonyl
compounds. Alternatively, the oxindole can be transformed to the 2-chloroindole with POCl3.
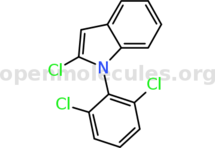
But asking others what you can do with some starting material without providing the entire list of available reagents is quite pointless.
PS: Please open topics without any references only in the Beginnings section. See the forum guidelines for more information.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
4-3-2016 at 09:36 |