ariep641514
Harmless

Posts: 6
Registered: 29-12-2015
Member Is Offline
Mood: No Mood
|
|
benzyl chloride syn
any body can give advice, its possible to synthesis benzyl chloride by reacting benzyl alcohol + NaCl + sulfuric acid?
sulfuric acid helps benzyl alcohol to release -OH by dehydrating them, and remain sulfuric acid react with NaCl to formy anhydrous HCl, that correct
or not?
i just try to get high yield benzyl chloride by reacting between benzyl alcohol with anhydrous HCl fumes generated in situ by salt and sulfuric acid
will do the job, but minimized distilation process
any another reaction will involved? thx for explanation
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
So for your reaction I'd have to think about the mechanism first to answer you what would happen.
I just checked the database for your desired reactant and product and it gave me some possibilities depending on what chemicals you have acess to.
So from Benzyl Alcohol Benzyl Chloride has been prepared with:
(leaving out some exotic ones like Hydrides...)
- Tin(IV)Chloride
- Thionyl Chloride
- Zirconiumoxychloride + LiCl
- Oxalylchloride
- Tungsten Hexachloride
- Bismuth(III)Chloride (with some loss)
- Vanadiumoxychloride
You might also check the following paper:
An Efficient Synthesis of 2-(Halogenomethyl)penems
I just had a look into it they seem to use CaCl2 there but I'm not sure about the other reagents used since I didn't want to read the whole paper for
it. If you need an information to any of the reagents mentioned above I can send you a link to the paper I just didn't want to copy all of them into
here.
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  |
So from Benzyl Alcohol Benzyl Chloride has been prepared with:
(leaving out some exotic ones like Hydrides...)
- Tin(IV)Chloride
- Thionyl Chloride
- Zirconiumoxychloride + LiCl
- Oxalylchloride
- Tungsten Hexachloride
- Bismuth(III)Chloride (with some loss)
- Vanadiumoxychloride
You might also check the following paper:
An Efficient Synthesis of 2-(Halogenomethyl)penems
|
...Or you know... just reacting it with hydrochloric acid.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Primary alcohols don't react very well with HCl. Unless you can distil off the reaction product, which here has a BP of 179 C.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ariep641514  |
sulfuric acid helps benzyl alcohol to release -OH by dehydrating them, and remain sulfuric acid react with NaCl to formy anhydrous HCl, that correct
or not?
|
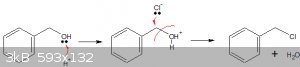
But as I wrote above, that reaction doesn't proceed very well, unless you remove the reaction product continuously, or use a catalyst.
[Edited on 29-12-2015 by blogfast25]
|
|
|
NitreRat
Harmless

Posts: 45
Registered: 22-1-2015
Location: Cyberspace
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  |
Primary alcohols don't react very well with HCl. Unless you can distil off the reaction product, which here has a BP of 179 C. |
Yes, but benzyl alcohol is a bit of an exception. Just mixing concentrated hydrochloric acid and benzyl alcohol then separating and drying the organic
layer, followed by fractional distillation will give to a decent yield of benzyl chloride.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NitreRat  |
Yes, but benzyl alcohol is a bit of an exception. Just mixing concentrated hydrochloric acid and benzyl alcohol then separating and drying the organic
layer, followed by fractional distillation will give to a decent yield of benzyl chloride. |
I didn't think the electron pushing effect of the phenyl group would reach that far.
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
It does, Blogfast. It has to be concentrated hydrochloric acid, but it works pretty well. I have done it a couple of times, here is an example with a reference.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Crowfjord  | | It does, Blogfast. It has to be concentrated hydrochloric acid, but it works pretty well. I have done it a couple of times, here is an example with a reference. |
In that case, in the spirit of OP's question, 40 % H2SO4 saturated with NaCl should probably work.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I have run the following reaction many times on much larger scale (20X) than described.
CHLORINATION OF BENZYL ALCOHOL WITH CONC. HCL [3]
2g benzyl alcohol and 6g concentrated hydrochloric acid were mixed and slowly heated. at 60°C the mixture separated into two layers. the yield of
benzyl chloride was 70% of the theoretical. When the alcohol and a large excess of the acid were mixed, the reaction took place at the room
temperature after a few minutes, and a theoretical yield of the chloride separated. Benzyl alcohol dissolves sparingly in hydrochloric acid of the
specific gravity 1.12. when the solution is warmed, benzyl chloride is formed. Benzyl bromide and benzyl iodide were prepared in the same way from the
corresponding acids.
[3] J F Norris, American Chemical Journal 38: 627-642 (1907)
The reaction does not work nearly so well with 31% hardware store acid. Benzyl chloride is formed but also dibenzyl ether is a significant by-product.
Far less ether is formed with 37% acid and yields of benzyl chloride are >90% after distillation. This observation may need to be taken into
consideration when using mixtures of sulfuric acid and NaCl.
AvB
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
First time?
The vapors from Benzyl Chloride are very lachrymatory. Like Satan's Farts, more or less.
Best to use a fume hood, or some other high volume ventilation system. Otherwise, the tears streaming down your face, can make it hard to work.
The Benzyl Carbonium Ion is pretty stable.....meaning reactive. Other alcohols may not be so amenable to substitution. Likewise, Benzyl Chloride
readily forms a Grignard reagent. Other Alkyl/Aryl Chlorides may be less compliant. Gotta use Bromides or Iodides, instead.
Apparently, Carbonium Ion, has become a dated expression. Gotta read up.
|
|
|
ariep641514
Harmless

Posts: 6
Registered: 29-12-2015
Member Is Offline
Mood: No Mood
|
|
wow, thx for quick reply, i already try simple measure 0,1 mol benzyl alcohol and NaCl , 0,2 mol sulfuric acid 96%. first i mixed benzyl alcohol and
nacl, then slowly drop sulfuric acid, first it getting warmed, then then start bubling and whole mixture became intensely yellow orange colored with
alot fume, which i believe its benzyl chloride are formed. but not brave to direct inhale that  , any advice for simple test benzyl chloride? now prepare for destilation, wonder for the yield , any advice for simple test benzyl chloride? now prepare for destilation, wonder for the yield 
btw for reacting benzyl alcohol with hcl already test and its worked, i just want to increase yield by directly reacting it with anhydrous hcl, since
if i use concentrated acid it use alot volume of hcl ( highest conc. only 36%) and more volume to distilate. Oh yeah fluorence thx for your idea using
CaCl2 it will more efficient if that work, i will try later 
|
|
|
Texium
|
Thread Moved
29-12-2015 at 20:44 |