alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
aromatic Organic chemistry problem/ diels alder
Hi,
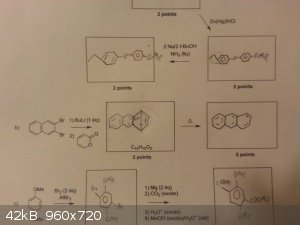
I had the following exercise to do (the b), I did it logically considering the reagents and formula given, but I have no god damn Idea how the hell
one equivalent of buli would remove both Br and allow the reagent to make a diels-Alder reaction with the reagent in 2). Is anyone able to explain
that / can tell me that i'm wrong and what should happen?
[Edited on 21-10-2015 by alexleyenda]
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It looks to be forming a reactive benzyne intermediate.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by alexleyenda  | but I have no god damn Idea how the hell one equivalent of buli would remove both Br and allow the reagent to make a diels-Alder reaction with the
reagent in 2). Is anyone able to explain that / can tell me that i'm wrong and what should happen?
|
It is possible,but I don't know how
http://www.orgsyn.org/demo.aspx?prep=v75p0201
https://en.wikipedia.org/wiki/Aryne#.5B4.2B2.5D_cycloadditio...
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
One equivalent of BuLi will lithiate the arene and generate BuBr as byproduct. Then eliminate LiBr to form the benzyne which is trapped by the diene.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
Oh I see, that is really an unusual reaction, thank you UC !
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|