| Pages:
1
2 |
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
DMP might be closer to hobbyist use than you think. IBX has been done: https://www.sciencemadness.org/whisper/viewthread.php?tid=12...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
well I will eat my....pipette Ok I give up Ok I give up
this is what I was looking for,exotic but easily obtainable if you know how
Now you have my mouth watering
[Edited on 5-2-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
I answered appropriately for the question that was asked. You made no mention of wanting esoteric or exotic reagents for the desired transformation.
You also mention that a Swern oxidation is probably the ideal for amateur chemists but you seem naïve of the fact that it is typically a cryogenic
reaction (at -78 *C) and whilst the reagents may be accessible by some means, the cooling is required to moderate the reaction between oxalyl chloride
and DMSO.
I also would not use DMP as a "routine" reagent for the oxidation of alcohols to aldehydes or ketones, as it is too expensive and relatively
difficult/time consuming to make (particularly as an amateur).
You make a comment about not wanting to use potassium dichromate because it is toxic then you go on to say that PCC/PDC would be an elusive dream from
the amateur chemist. You may (or may not, depending on how you feel about hexavalent chromium) find the attached paper of interest.
I also stand by my previous comment - I've attached a fairly detailed e-book on alcohol oxidation for you to get started on. Perhaps you should read
some more real chemistry rather than "how to cook meth" books.
Attachment: Potassium chlorochromate on solid support.pdf (530kB)
This file has been downloaded 630 times
Attachment: Oxidation Of Alcohols To Aldehydes And Ketones.pdf (2.7MB)
This file has been downloaded 15475 times
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  |
You also mention that a Swern oxidation is probably the ideal for amateur chemists but you seem naïve of the fact that it is typically a cryogenic
reaction (at -78 *C) and whilst the reagents may be accessible by some means, the cooling is required to moderate the reaction between oxalyl chloride
and DMSO |
come on man,maintaining such a low temperature is not that hard.cooks have been doing it all the time using dry ice/acetone to liquify NH3
for the birch reduction before the shake-n-bake method was invented.I later learnt that the swern oxidation releases CO and dimethyl sulphide and both
are toxic gases
| Quote: | | I also would not use DMP as a "routine" reagent for the oxidation of alcohols to aldehydes or ketones, as it is too expensive and relatively
difficult/time consuming to make (particularly as an amateur) |
although I was impressed by chemo's post on IBX,I noted the fact that anthranilic acid was used as the starting chemical for iodobenzoic acid
| Quote: | | You make a comment about not wanting to use potassium dichromate because it is toxic then you go on to say that PCC/PDC would be an elusive dream from
the amateur chemist |
yes,PCC/PDC are dangerous without a doubt,but their pros outweigh the cons.then I can say that even diazomethane is dangerous.Does that mean people
don't use it.Anyways I am never a supporter of these selective reagents.I prefer OTC ones.Also how many home chemists have been able to get their
hands on some PDC first to be able to worry about its toxicity
| Quote: | | I also stand by my previous comment - I've attached a fairly detailed e-book on alcohol oxidation for you to get started on. Perhaps you should read
some more real chemistry rather than "how to cook meth" books. |
I have read that book,and many others.I think you should stop classifying books by name and see what is inside them first.Incase you haven't
noticed,most of the reactions given in textbooks are impractical(phenol to benzene using Zn dust) and the reagents are expensive
too(PCC,PDC).Cookbooks on the other hand use OTC chemicals and equipments.
Quote: Originally posted by CuReUS  |
The only ones that came to my mind was the oppenauer oxidation,lead tetracetate(but this might methylate thiopene),lead nitrate(not sure about this
one,but I read that it was an alternative to hexamine for the sommlet reaction),potassium ferricyanide(I am not sure about this one too)
|
I was wrong about the lead tetraacetate.It will only methylate if you boil the substrate with Pb(OAc)4 in the presence of GAA .At room temp
it will oxidise alcohols to aldehydes and ketones]
[Edited on 6-2-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
come on man,maintaining such a low temperature is not that hard.cooks have been doing it all the time using dry ice/acetone to liquify NH3
for the birch reduction before the shake-n-bake method was invented.I later learnt that the swern oxidation releases CO and dimethyl sulphide and both
are toxic gases
|
Dry ice is not available OTC in many locales, and as such many amatuers have a hard time getting hold of it. I was under the impression that many
cooks stole their anhydrous ammonia from farm tanks, where it is readily dispensed in a liquid state. Dimethyl sulfide is a volatile liquid by the
way, not a gas. Its not too toxic but it does stink alot. I've done a Parikh Doering oxidation several times on 1 kg scale, and although the reaction
was complete in several hours, it was a couple days work to perform the isolation. The odour of dimethylsulfide was still noticeable after four more
synthetic stages, despite drying in a vac oven and chromatographic purification of the final product.
Quote: Originally posted by CuReUS  |
yes,PCC/PDC are dangerous without a doubt,but their pros outweigh the cons.then I can say that even diazomethane is dangerous.Does that mean people
don't use it.Anyways I am never a supporter of these selective reagents.I prefer OTC ones.Also how many home chemists have been able to get their
hands on some PDC first to be able to worry about its toxicity
|
I agree that many may consider PCC/PDC out of reach, but I suspect the need for pyridine is what causes the difficulty. Other members have
demonstrated the preparation of pyridine from nicotinic acid, and whilst it may be cumbersome to produce several hundred grams, it would be
satisfactory for preparation of a few grams of the chromate reagents. This is reasonable for research purposes.
Whilst OTC materials are preferable, the selectivity/ability provided by some reagents justifies the efforts required to prepare them in-house (excuse
the pun). When working with relatively complex molecules, these reagents become a necessity rather than a luxury.
Oh you did, did you? Perhaps you didn't have the perseverence to reach pp. 341-342 where the Stevens oxidation is detailed, along with a preparative
method. If you had read the book as you claim, AvBaeyer's post would not have been so suprising.
Quote: Originally posted by CuReUS  |
I think you should stop classifying books by name and see what is inside them first.Incase you haven't noticed,most of the reactions given in
textbooks are impractical(phenol to benzene using Zn dust) and the reagents are expensive too(PCC,PDC).Cookbooks on the other hand use OTC chemicals
and equipments.
|
Selective reagents are expensive, but they're intended for research purposes where you aren't often using grams at a time. Again, OTC
is a very location (and circumstance) specific term, and what is freely available for some may be heavily restricted for many.
Quote: Originally posted by CuReUS  |
I was wrong about the lead tetraacetate.It will only methylate if you boil the substrate with Pb(OAc)4 in the presence of GAA .At room temp
it will oxidise alcohols to aldehydes and ketones |
What does it matter? Lead tetraacetate is not a reagent you should recourse to without good reason. It is not OTC and suffers hydrolysis in storage.
On top of that, its pretty toxic, certainly for something like the OPs question which could be done under the Stevens conditions using benign
reagents.
[Edited on 9-2-2015 by DJF90]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  |
Dry ice is not available OTC in many locales, and as such many amatuers have a hard time getting hold of it. |
don't you get it in walmart.Otherwise just ask the counter guy at dippin dots or ben and jerry's the next time you go there.
| Quote: | | I was under the impression that many cooks stole their anhydrous ammonia from farm tanks, where it is readily dispensed in a liquid state.
|
https://www.erowid.org/archive/rhodium/chemistry/birch.mrcle...
| Quote: | | ]Lead tetraacetate is not a reagent you should recourse to without good reason. It is not OTC |
https://www.erowid.org/archive/rhodium/chemistry/lead.tetraa...
I was searching for OTC sources of red lead and this is what I found
http://www.sciencemadness.org/talk/viewthread.php?tid=53527#...
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
As I said before, availability of dry ice depends on location. There is no OTC source here in the UK, but it can be ordered online at a relatively
high price. If you can get it at any supermarket in the states, then good for you. However I try to consider that everyone may not have the same
potential resources as myself.
It would be several steps to produce PCC/PDC but you'd benefit from several intermediates which have additional uses. Everything needed has been
documented on this forum at some point or another, but not necessarily tied together. There are alternative reagents/conditions as outlined in the
book I provided. Some will be easier to make use of than others.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
I find it interesting how dry ice is sold in so many places in the USA. Here in Canada I need to go to places like Air Liquide or Praxair (they have
store fronts) but the dry ice is pretty pricey.
Note to self: Tare the damned flask.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by Mailinmypocket  | | I find it interesting how dry ice is sold in so many places in the USA. Here in Canada I need to go to places like Air Liquide or Praxair (they have
store fronts) but the dry ice is pretty pricey. |
There is a fish market about right around the corner from my house where I can purchase dry ice, not to mention the grocery store, which is pretty
much right behind my house.
[Edited on 9-2-2015 by Loptr]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
what about using fenton's reagent to oxidise the secondary alcohol ? is fenton's reagent more stronger than just H2O2 ?
Even I was worrying about this.the acetylacetone method might be the only way to make the ketone,the B-keto ester method may not work,the ring being
too electron rich for the methylene carbon to attack it
http://en.wikipedia.org/wiki/Acetoacetic_ester_synthesis#med...
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Fenton's reagent is aggressive and best saved for cleaning glassware/disposing of waste organics (BromicAcid has used it for this).
I see two potential routes to the desired compound (without thinking hard about it):
1) Metallation of thiophene and alkylation with propylene oxide. This should give the alcohol, which can be oxidised up using any suitable reagent
(see the book I posted before).
2) Knoevanagel condensation of thiophene-2-carboxaldehyde with nitroethane to obtain the nitroalkene, which may be treated with Fe/HCl to afford the
ketone. I'm aware of a similar transformation to obtain 1-naphthylacetone in the literature.
EDIT: Just realised route #1 was already suggested by Darkstar. Sorry about that.
[Edited on 10-2-2015 by DJF90]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Fenton's reagent is aggressive and best saved for cleaning glassware/disposing of waste organics |
DFJ90,although I respect you a lot,I feel that is a very wrong thing to say.Its like saying petroleum should be only used as fuel and not for anything
else
| Quote: | | 1) Metallation of thiophene and alkylation with propylene oxide. This should give the alcohol, which can be oxidised up using any suitable reagent.
|
what about this?
allyl bromide is cheaper than propylene oxide and for the wacker,you will need ~1g of PdCl2 and it can be reused again.
| Quote: | | 2) Knoevanagel condensation of thiophene-2-carboxaldehyde with nitroethane to obtain the nitroalkene, which may be treated with Fe/HCl to afford the
ketone |
this route is not helpful as the OP's starting compound is 2-iodothiophene
[Edited on 11-2-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Sure, Fenton's reagent probably has some synthetic use, but I've never seen anything just browsing the literature. Because it is reliant on he
generation of *OH radicals anongside Fe(IV), it is a very aggresive oxidant as I mentioned.
Propylene oxide can be made from propylene glycol, which is piss cheap. Sure, it adds another step, but allyl bromide isn't exactly OTC either and
would require two reactions to prepare.
As for thiophene-2-carboxaldehyde, it can be made via metallation (e.g. grignard formation) and reaction with DMF or N-methylformanilide (Bouveault
aldehyde synthesis).
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DJF90  | | Sure, Fenton's reagent probably has some synthetic use, but I've never seen anything just browsing the literature. |
I am attaching some files ,enjoy
how ?
| Quote: | | As for thiophene-2-carboxaldehyde, it can be made via metallation (e.g. grignard formation) and reaction with DMF or N-methylformanilide (Bouveault
aldehyde synthesis). |
now that's a beautiful reaction
but I think I have found a good reaction.No need of even iodothiopene,plain thiopene might work.this chemical is needed
http://en.wikipedia.org/wiki/Manganese%28III%29_acetate
here is an electrochemical prep,from here
http://onlinelibrary.wiley.com/doi/10.1002/hlca.201100105/ab...
| Quote: | Electrochemical Preparation of Mn(OAc)3
Mn(OAc)3 ·2H2O was obtained in an optimized bipolar
packed-bed reactor by the electrochemical method described in [14]. However, to obtain Mn(OAc)3, this reactor was modified to a semi-pilot scale.
Therefore, a reactor consisting of a glass tube (inner diameter,52 mm) having 40 rows of graphite rings inside was designed. Each horizontal row in
the reactor had seven graphite rings which were 14 mm long, 16 mm in outer diameter, and 10 mm in inner diameter.
Each horizontal row was insulated from each other by placing a Teflon net with 1-mm thickness between the rows. Applying a potential to the reactor
through two graphite rods, 10 cm in lenght and 6 mm in diameter, placed top and bottom of the reactor, Mn(OAc)2was oxidized to Mn(OAc)3by
electrolysis.
For this, a soln. containing 100 mm Mn(OAc)2 and 400 mm AcONa was prepared from 95% AcOH. During
applying 180 V cell potential supplied by 500 V/1A dc power unit to the reactor, this colorless soln. was added to the reactor continuously with a
dosage pump with 2.5 l/h flow rate. The red-brown soln. collected from the bottom of the reactor was recycled through the reactor under the same
condition. The obtained soln. was kept in a closed glass container for 3 – 4 d, until Mn(OAc)3 completely precipitated,
and the color of the soln. disappeared. The red-brown precipitate was filtered through a sintered funnel (diameter, 120 mm) with porosity 4 and washed
with glacial AcOH (2X100 ml) and than Et2O (2X100 ml). The obtained solid was dried under vacuum in a desiccator containing P2O5 for one week.
Yield: 120 g (90%) |
or see this
| Quote: | | "commercially available dihydrate is easily prepared by the reaction of manganese(II)acetate with KMnO4 in acetic acid at reflux.the
anhydrous form is prepared in acetic acid and acetic anhydride" |
EDIT:
I forgot to attach the real pdf itself. ,the one with the Mn(OAc)4
reaction.but I see now that ionic liquids will be needed for the reaction.Is TEMPO a substitute for ionic liquids ? ,the one with the Mn(OAc)4
reaction.but I see now that ionic liquids will be needed for the reaction.Is TEMPO a substitute for ionic liquids ?
Attachment: alcohol.oxidation.iron-h2o2.pdf (258kB)
This file has been downloaded 555 times
Attachment: DCM(pg 5) as solvent for peroxide oxidation.pdf (785kB)
This file has been downloaded 552 times
Attachment: Hydrogen-Peroxide-as-an-Oxidant-for-Organic-Reactions (1).pdf (263kB)
This file has been downloaded 697 times
Attachment: snider2001.pdf (62kB)
This file has been downloaded 662 times
Attachment: taming the fenton reagent.pdf (2MB)
This file has been downloaded 663 times
Attachment: synthesis of arylacetones.pdf (56kB)
This file has been downloaded 533 times
[Edited on 16-2-2015 by CuReUS]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Like DJF90 already pointed out, you simply asked for "potential oxidizing agents." You made no specific mention about wanting a bunch of exotic or
novel methods/reagents. Given the way the question was worded and your surprise when AvBaeyer claimed that secondary alcohols can be oxidized to
ketones using hypochlorites under acidic conditions, I kind of got the impression that you weren't already familiar with the usual oxidizers. (which,
admittedly, did seem a little strange, as many of your posts clearly demonstrate an understanding of chemistry more advanced than that of someone who
would honestly need to ask)
| Quote: |
No one nowadays wants to work with chromium because its too toxic |
You're telling me there's not a single person out there who's okay with using chromium oxidizers these days? Come on, man, it's hexavalent chromium,
not dimethylmercury. It's not THAT toxic. Besides, when it comes to drug cooks -- and I stand by my assumption that the OP's question is
cookery-related -- they usually seem far more concerned about whether or not the reagent actually works, and, if so, how easy it is to obtain.
Toxicity rarely ever seems to be at the top of their list of concerns. If it were, they'd stop using aluminum amalgam for their imine and nitro group
reductions since it involves toxic mercury(II) salts and generates elemental mercury waste.
| Quote: |
permaganate had been put on the schedule 2 list |
Permanganates like KMnO4 and NaMnO4 are List II, not Schedule II. These are two very different lists from a
legal standpoint (purchase, possession, manufacture etc). For example, acetic anhydride, acetone, KMnO4 and toluene are List II. Schedule
II substances, however, are things like cocaine, d-methamphetamine, morphine, oxycodone and PCP, as well as precursors like phenylacetone and a couple
for PCP. Compared to those substances, List II chemicals aren't even remotely "controlled" or difficult to obtain.
| Quote: |
MnO2 is generally suitable for allylic and benzylic hydroxyl groups and thiopenyl propan-2-ol is neither. |
Activated MnO2 will oxidize saturated alcohols under neutral conditions. The reaction would take longer than with an allylic or benzylic
alcohol, and the yields wouldn't be as great, but it would work.
| Quote: |
the H2O2/HCOOH method creates a mess |
Who said anything about performic acid? If you tried to oxidize a secondary alcohol that way, you'd probably end up with an ester. Peroxy acids react
with ketones via the Baeyer–Villiger oxidation.
What I was referring to is using aqueous H2O2 with a metal catalyst under phase-transfer conditions. This is one of the
greenest, most environmentally-friendly oxidation methods there are. There's no mess, the work-up is extremely simple, the yields are very good, and
the only byproduct is water.
| Quote: |
what about using fenton's reagent to oxidise the secondary alcohol ? |
I haven't looked at all of the .pdf files you've attached yet (I've seen most of them judging by their titles anyway), but, as you probably know by
now, Fenton’s reagent can indeed be used to oxidize secondary alcohols. With that said, if you haven't already done so, I highly recommend you look
into Fe(III)-catalyzed H2O2 oxidations. FeBr3 seems to work well. The oxidations can be done under
organic-solvent-free conditions at room temperature with high yields (like 90%+), and the reaction doesn't require a metal complex or phase-transfer
catalyst. Check out that first .pdf you attached if you haven't already.
[Edited on 2-16-2015 by Darkstar]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
| Quote: | | As for thiophene-2-carboxaldehyde, it can be made via metallation (e.g. grignard formation) and reaction with DMF or N-methylformanilide (Bouveault
aldehyde synthesis). |
now that's a beautiful reaction
|
It did not strike my mind then,but coming to think of it now,thiopene-2-aldehyde can be made by refluxing with CHCl3 and NaOH
on a side note,how would you synthesize this compound
[Edited on 19-2-2015 by CuReUS]
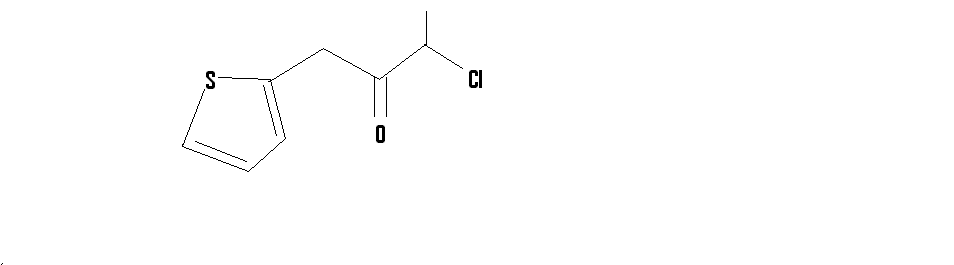
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
CuReUS, do us all a favour and get a decent structure drawing software. The free one of choice used to be ACD chemsketch, but more recently
MarvinSketch by ChemAxon is in my experience much nicer to use. You can download the Marvin Beans software package at
http://www.chemaxon.com/download/marvin-suite/
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
I have the ACD chemsketch,but the problem is if I upload the file,other people will not be able to see it because the file format is different
anyways,the compound that I have drawn can be clearly understood,so try to create,not complain
Is thiopene electron rich enough for a duff reaction ?
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Well, if you'd look carefully at your fine software, you'd see it has the capability to save chemical structures as *.gif, and *.png... in any event, you might be interested in US2543318.
sparky (~_~)
[Edited on 20-2-2015 by sparkgap]
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Thanks for the patent,but I don't see much difference in that and doing a duff reaction
for the latter,you use hexamine which you make from NH3 and HCHO and for the former,you react HCHO with an ammonium salt along with
thiopene
so we can safely conclude that duff reaction can be used to formylate thiopene
I found this website for drawing structures,and it generates the image source code immediately,which can be copy-pasted here.The only problem is that
the image formed is messed up .Does anyone know why ?so my compound appears like
this(its an alpha-halo ketone) .Does anyone know why ?so my compound appears like
this(its an alpha-halo ketone)

http://www.webqc.org/moleculareditor.php
[Edited on 21-2-2015 by CuReUS]
|
|
|
| Pages:
1
2 |