chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
Self-made reflux
Sometimes one needs to boil small quantitie of liquid for long time. This is not as easy as it may seem because of liquid losses by evaporation. Loss
of liquid is not always a problem - for example one may compensate evaporation of water by adding some more but this is not of any help if substances
that become lost are your desired reaction products.
These problems can be solved by using reflux condensers. Dedicated reflux condensers can be bought from glassware sellers but you can make your own
especially if you prefer to experiment with small quantities.
Probably simplest reflux condenser would be if we place cold object over boiling liquid. Vapour condenses on cold surface and flows back into liquid.
This would be rather good way if only our cold object would remain cold as long as we want.
This can be done by palcing stopperd test tube over boiling liquid and driving cold water through it. If vapour can not escape without contact with
cold surface then we have quite useable reflux condenser.
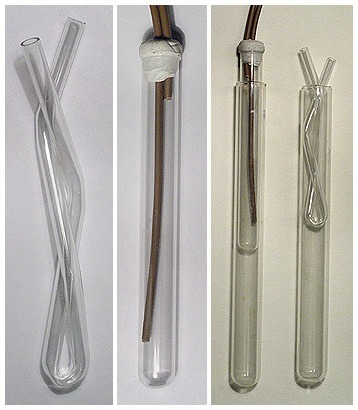
Another way is to take class tube aproximately 25 cm long and to bend it from center part so that to get incredible narrow U tube. I used glass tube
with 6mm outer diameter. Central bent was rather hard to make. I had to try it 4 times (and spent 4 tubes) before i got "U" that was thin
enough to fit into test tube with 15mm inner diameter. Two or three additional bends will make it coil-like for increased efficiency. Cheap resin
tubing can be used to supply such condenser with cold tapwater. Such condenser can be placed onto neck of suitable test tube. Small quantity of liquid
can boiled then for many hours without noticeable loss. Of course you have to use boiling stones and water (or oil) bath is good to ensure quiet
boiling.
If condenser is intended to use solely with organic solvents then it can be made from metal tube. More complex coil shapes can probably even be placed
in neck of common boiling flasks.
Condensers of this kind are good for many organic experiments. Synthesisizing of esters is good example.
[Edited on 2-12-2005 by chromium]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
How does this look? I want the look to be clean, standardized, and not too fancy. I made some minor changes to your text, Chromium, but it is mainly
as received.
http://www.sciencemadness.org/scipics/improvised_reflux.pdf
PGP Key and corresponding e-mail address
|
|
|
Thomas Winwood
Harmless

Posts: 43
Registered: 16-12-2004
Location: United Kingdom of Great Boredom
Member Is Offline
Mood: Anhydrous
|
|
Synthesisizing? =P
Nice though.
I\'ve been told having a sig is banned, despite the facility being available. Um...contradiction?
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
I like this pdf. Its good you bothered to make some changes to text as my english is not perfect.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Air cooled reflux condenser
I have the difficulty of lacking a water supply in my lab, therefor i tried something different.....air cooling.
My prototype consists of an ordinary CPU-cooler(copper-heat sink + silent fan copper silent) which is attached to an eloxated heat-sink with lamella pointing inside the flask.
I have tested such a setup for use with non-corrosive solvents such as toluene or acetone........it works!
You can calculate from the performance of these heat-sinks that it would be best to incorporate 2-3 coolers for a normal reflux operation.
Ok, this is somewhat crazy and many would prefer to use just a high copper tube for air cooling, but it is the small size of the whole setup that
fascinates me...
[Edited on 5-12-2005 by BASF]
|
|
|
Axt
National Hazard
   
Posts: 794
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Polverone
How does this look? I want the look to be clean, standardized, and not too fancy. |
Personally I'd reduce the size of the headings and stretch the header thinner so it doesnt look so blocky. It may be an idea to use different font for
peoples usenames, as some names within the context of a paragraph can cause confusion. Below I used "shorthand" font to distinguish the usename from
the content. Kinda makes it look like it was signed in to suggest someones name.
<center><img src="http://www.sciencemadness.org/scipics/axt/scimadheader.jpg"></center>
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Axt
Personally I'd reduce the size of the headings and stretch the header thinner so it doesnt look so blocky. It may be an idea to use different font for
peoples usenames, as some names within the context of a paragraph can cause confusion. Below I used "shorthand" font to distinguish the usename from
the content. Kinda makes it look like it was signed in to suggest someones name. |
I think that's unnecessary. It should be obvious that if the author is referenced in the work, but I've always written my procedures to avoid the
usage or reference to myself.
Just on a side note, the font that you used for Chromium (the name) doesn't suit my tastes too much; it seems too informal and takes away from the
"professional feel" of the entire article. If it really is necessary, perhaps not a font change is required, but simply putting the "by Chromium" in
smaller font on a second line will suffice.
I do agree wtih stretching the header, perhaps making it longer and moving it up so that it is positioned higher.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
I have updated the PDF with a wider header and a different appearance for the author name.
PGP Key and corresponding e-mail address
|
|
|