cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Suggestions for Crystal Growing
Hello everyone!
I've been a member here a while; I've browsing occasionally and reading.
I'm interested in growing crystals. I'm looking for suggestions of more things to grow crystals of.
Preferably, I'd like to make the chemicals myself because I find syenthesizing things really fun.
I've got:
HCl
Citric Acid
Ascorbic Acid
Vinegar
NH4H2PO4
Copper
NaHCO3
KC4H5O6
Cu(CH3COO)2
Potassium Alum
Rochelle Salt
Iron
MgSO4
CaCO3
NaCl
Sucrose
I'm considering buying CuSO4.
I've grown crystals of monoammonium phosphate, alum, Rochelle salt, and copper acetate.
I'd especially be interested in double salts with copper.
Also, I've had troubles keepig microbes out of some of my growing solutions, especially Rochelle salt. I've got some filter paper coming. Should I
just filter it to keep them out?
Thank you for any suggestions!
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Definitely get some copper sulfate. It's the only crystal I've grown so far, but it sure is a pretty blue.
As for microbes, make sure your glassware is very clean. Use very hot water, preferably boiling, a small amount of soap, and be sure to rinse well.
Also consider rinsing with alcohol. Basically treat all utensils as if you were going to eat from them. Also, use distilled water to make your
solutions, and cover them while the crystals are growing. Use a paper towel if you want faster evaporation, or a watchglass or a loose cover of
aluminum foil for slower evaporation. Filtration will probably not remove all the microbes already growing in a solution, you've got to kill them with
heat or chemicals. Boiling the solution for ten minutes is probably the best way.
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
I have before mentioned growing nacl crystals from superheated supersaturated solutions obtained by careful partial neutralisation of hot conc caustic
solutions using hcl.
Fantastic cubic cities grow.
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
If you're willing to shell out some cash, try buying terbium metal from Metallium (it's expensive, $32 for 5 g) but you can make fluorescent crystals
of terbium nitrate or chloride. Europium ($50 for 5 g) can also be used. The only problem is that you will need a dessicator bag, but it's definitely
worth doing!
You can try nickel sulfate, but it's hard to get right. If you do, however, the crystals are really nice. Alum can be used to grow a transparent layer
over chrome alum.
Copper sulfate is an excellent choice. Copper chloride is as well - you get needle-like crystals instead.
And don't forget crystals of bismuth! Add a little bit of tin if you don't want the colorful oxide layer on top.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
jock88
National Hazard
   
Posts: 505
Registered: 13-12-2012
Member Is Offline
Mood: No Mood
|
|
Try growing copper 'trees' from a copper sulphate solution.
This may work for other metals as well. Silver will definately work (expensive).
Would it work for iron does anyone know?
Varying the values of r1 and r2 give varying results.
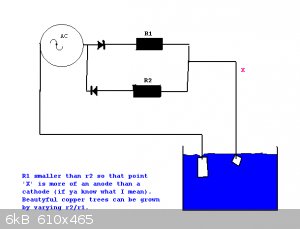
|
|
|
Texium
Administrator
       
Posts: 4593
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Hello fellow Austinite! 
One compound that I'd recommend trying is potassium nitrate. It makes very nice, long crystals, and you can buy it at Lowe's as Spectracide stump
remover. Recrystallizing the stump remover is useful as well as nice looking, since it also contains some impurities that are removed in the process.
Copper compounds are always nice of course, and copper sulfate is one of the best. I've crystallized copper chloride before too, and like
Brain&Force said, it makes needle shaped crystals that are very nice looking, but they're also very fragile and don't grow very large. Iron(II)
sulfate has a very nice color and interesting texture, and can be easily made if you get some sulfuric acid. It must be crystallized from a quite
acidic solution or protected from the air though, because otherwise it will oxidize to iron(III) sulfate and iron(III) oxide, which is an awful mess.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I've boiled solutions of mixed copper and nickel acetates to very near dryness to obtain a beautiful blue-green crystalline powder that glitters all
over. I've also heard that large crystals of copper(II) acetate can be made using concentrated hydrogen peroxide and glacial acetic acid from copper,
though that is beyond some of us, resource-wise. I currently have a crystal of copper(II) sulfate about the size of the end of my thumb. It's very
easy to grow crystals of it and very satisfying to see the results; I recommend you make your own if you can get some sulfuric acid(car battery
electrolyte works great for this, as you don't need it very concentrated). Sodium acetate makes long, hard, well-formed crystals that kind of look
like transparent selenite, they look pretty nice if you carefully grow them to a larger size. Finally, I would look into growing a crystal garden
using sodium silicate if you can find some, or to try precipitating tetrammine copper(II) sulfate crystals later on. The last two can just be google
searched.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
When you get tired if growing ordinary soluble salts you might try the hydrothermal process for crystal growing of sparingly soluble compounds.
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Have you done this yourself? I don't know how much an autoclave costs, but I assume it's a lot.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
The autoclave setup is only needed for sensu stricto hydrothermal crystallizations. You can do something similar at room temperature.
For example, a narrow tank, bottle or flask filled with water with a small mound of the material at one end. That end is warmed very slightly (maybe
a glasshouse seed-warming pad) and some of the material dissolves. The warm solution rises, travels along the surface, and sinks at the other end as
it cools. That is where you have something rough that crystals can grow on, and when you get a decent seed crystal you can drop it in on a thread or
wire.
You cannot do quartz at room temperature, but you might be able to do copper phosphate, or sparingly soluble organic salts such as calcium oxalate.
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Paddywhacker  | The autoclave setup is only needed for sensu stricto hydrothermal crystallizations. You can do something similar at room temperature.
For example, a narrow tank, bottle or flask filled with water with a small mound of the material at one end. That end is warmed very slightly (maybe
a glasshouse seed-warming pad) and some of the material dissolves. The warm solution rises, travels along the surface, and sinks at the other end as
it cools. That is where you have something rough that crystals can grow on, and when you get a decent seed crystal you can drop it in on a thread or
wire.
You cannot do quartz at room temperature, but you might be able to do copper phosphate, or sparingly soluble organic salts such as calcium oxalate.
|
Thanks for this, but I still wonder if you have actually done this, or are simply speculating.
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
I used a Thiele tube, tilted on an angle, as an apparatus, but didn't have any luck trying to grow Prussian Blue.
|
|
|
cpman
Harmless

Posts: 36
Registered: 9-12-2013
Location: Austin, TX
Member Is Offline
Mood: No Mood
|
|
Thanks for the suggestions!
I'm going to build a temperature controlled tank to grow crystals. I've already got everything but the heating elements. That way, they'll be
insulated from temperature changes. In the past, I've had my crystal growth go awry with evaporation because of temperature changes in the house...
I've now got a several months worth of projects to try!
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
copper acetate, but it must be perfectly steady evaporation of solution.. had some standing on my radiator with inverted computer fan blowing onto it,
managed to fuck up the radiator and it went out for a few hours, bam. billions of tiny little crystals everywhere.. totally destroyed my attempt on
making larger crystals
otherwise CuCl2, FeAc and potassium ferrioxalate
benzoic acid perhaps
KClO3 solution slowly cooling down forms some really nice ultra thin square pieces, projecting light all around the solution as it cools down, quite
the show if you have sunlight to put through it
NaClO3 by my experience can form nice large rectangular crystals, they are really perfectly square, 90*
not sure if you can make crystals out of it, but NaOH 3:1 molar ratio with MnO2 if heated very well will form blue Na3MnO4, which is interesting
enough by itself
but for crystal growth i can only reccomend using a computer fan to drag water out from beaker or up from dish or whatever you use for it
|
|
|
knowledgevschaos
Harmless

Posts: 41
Registered: 9-8-2023
Location: Sci-Hub and the hardware store
Member Is Offline
Mood: Hungry for information
|
|
Magnesium sulfate, while not as good as some others, can definitely make some very good crystals.
It is so soluble, especially in hot water, that it's crystals will grow faster than almost anything else. By cooling a saturated solution, a crystal
the size of your palm can be grown practically overnight. The only problems are that it may not be as perfect or transparent as slower growing
crystals, and it will dehydrate in air and turn white over time. It's definitely a good start though.
Also, try these:
https://dmishin.github.io/crystals/
This site recommends using iodine to kill microbes in solution, and has tonnes of different compounds.
|
|
|