aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Noob marches on
Just thought i'd share my progress since i started 13 days ago.
First of all there seems to be a lot more stuff in my shed, and a bit less money in the bank.
Theory :-
Atomic structure/electron shell configurations
Bonding types
Atomic weight/Molarity
Ideal Gas Law
Techniques tried :-
Reacting stuff in a pot without damaging or losing body parts
Reacting stuff in a pot and catching the gas evolved
Electrolysis
Filtering
Titration
Crystallisation/recrystallisation
Substances made (as far as i can tell) :-
Copper Sulphate
Tetra Cloro Cuprate*
Tetra Ammine Copper(II) Sulphate*
Copper (II) Hydrogen Carbonate*
Copper Nitrate
Ammonia
Ferric Acetate
Aluminium Sulphate
Copper Acetate (failed - could not decide which bit was the product)
Sodium Aluminate (failed and broke the flask trying to remove the grey sludge)
Next things to try will be :-
Distillation
Intensive Cleaning Up and/or buy new glassware
All been great fun so far !
Suggestions on 'What To Do Next' always gratefully received.
(items marked with * were made using some of the huge volume of Copper Sulphate solution)
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Just an fyi, metal hydrogen carbonates are not stable. I presume you reacted copper sulfate and sodium bicarbonate in which case the following
reaction occurred : 2NaHCO3 + CuSO4 --> CuCO3 + H2O + CO2
The copper carbonate formed quickly hydrolyzes into basic copper carbonate : 2CuCO3 + H2O -->
Cu2CO3(OH)2 + CO2
[Edited on 7-4-2014 by gdflp]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
That's exactly what i did !
So it's CuCO3 Copper (II) Carbonate then ?
That was the first one to work properly, as in it's now a dry turqoise powder in a jar.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Yes only group I bicarbonates are stable in solid form, some other bicarbonates are stable in solution only but decompose upon crystallization into
the carbonate.
[Edited on 7-4-2014 by gdflp]
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
As soon as you mix the solutions, copper bicarbonate is formed, but it decomposes spontaneously to the carbonate.
|
|
|
Texium
Administrator
       
Posts: 4587
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It will form as the basic carbonate though, getting the pure carbonate takes a lot more effort and processes that are impractical in most home labs.
What you have is Cu2CO3(OH)2, basic copper carbonate.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
For some related fun, you could react (Sodium free!) Copper carbonate with food grade benzoic acid dissolved into a minimum amount of distilled water
to yield Carbon dioxide (bubbles out of solution & escapes) and Copper benzoate (insoluble, precipitates)-
Copper benzoate
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Two simple but nice experiments you could try are:
1. Synthesis of ammoniumiron(III)sulphate. Grows large lilac crystals. Environment should not be too dry to keep them.
2. Synthesis of hexamolybdatochromate(III). This is a beautiful chameleon reaction going from a bluish starting solution to green as soon as mixed.

Then through light green/yellow, grey/brownish, purple to a final pink solution, out of which crystals form on longer standing.
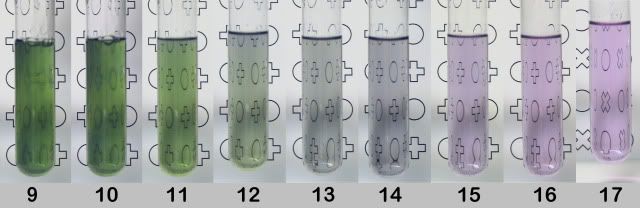
The exact colours you will encounter depend on the condition details.
The final product, (NH4)3H6[Cr(MoO4)6]·7H2O is obtained by mixing stoichiometric amounts of chrome alum and ammoniumheptamolybdate (molar 7:6).
[Edited on 7-4-2014 by Bezaleel]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Many thanks. I will re-label the jar ...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Bert  | For some related fun, you could react (Sodium free!) Copper carbonate with food grade benzoic acid dissolved into a minimum amount of distilled water
to yield Carbon dioxide (bubbles out of solution & escapes) and Copper benzoate (insoluble, precipitates)-
Copper benzoate
|
Sounds like Fun. Hopefully not TOO much fun ....
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  |
1. Synthesis of ammoniumiron(III)sulphate. Grows large lilac crystals. Environment should not be too dry to keep them.
2. Synthesis of hexamolybdatochromate(III). This is a beautiful chameleon reaction going from a bluish starting solution to green as soon as mixed.
... The exact colours you will encounter depend on the condition details.
The final product, (NH4)3H6[Cr(MoO4)6]·7H2O is obtained by mixing stoichiometric amounts of chrome alum and ammoniumheptamolybdate (molar 7:6).
|
Wicked ! I will order the stuff for that. Cheers !
|
|
|
gardul
HAZARD TO TEH CATZ!
  
Posts: 256
Registered: 18-10-2014
Location: Under the Sun in a beaker
Member Is Offline
Mood: Vivified!
|
|
Boric acid and methanol alcohol. Add some copper and you get an interesting Blue base and green flame. Just be careful. If you need to know how to
make boric acid I or someone else here i am sure will be happily to show you. it is rather simple.
|
|
|