Finnnicus
Hazard to Others
  
Posts: 342
Registered: 22-3-2013
Member Is Offline
|
|
Ozone solubilities - Chlorocarbons
The only reference I could find for this is the wiki page which states:
"Ozone is a pale blue gas, slightly soluble in water and much more soluble in inert non-polar solvents such as carbon tetrachloride or
fluorocarbons, where it forms a blue solution."
Does this have any truth to it? Any one have any references? Anybody with an O3 generator willing to try it?
Also, would an ozone solution be an oxidizing agent similar to hydrogen peroxide?
|
|
|
testimento
Hazard to Others
  
Posts: 351
Registered: 10-6-2013
Member Is Offline
Mood: No Mood
|
|
What I've read, ozone should be soluble in tetrachloride and sulfuric acid, but the solubilities are still rather limited, speaking of few grams per
liter maximum. Yes, it can be used as an oxidant, and according to my info, it will generate NH4NO3 from NH3(+H2O?), make SO3 of SO2, create lead
oxide in situ and corrode several materials.
Im planning on building electrolytic ozone cell, similar to chlor-alkali, but using only pure water with 0.5-1% NaOH as electrolyte: it should
generate up to 30% O3 with O2 at cathode and H2 from anode.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<a href="http://en.wikipedia.org/wiki/Ozone" target="_blank"> | Quote: | <strong>Physical properties</strong><hr /><a href="http://en.wikipedia.org/wiki/Ozone" target="_blank">Ozone</a> <img
src="../scipics/_wiki.png" /> is a pale blue gas, slightly soluble in water and much more soluble in inert non-polar solvents such as <a
href="http://en.wikipedia.org/wiki/Carbon_tetrachloride" target="_blank">carbon tetrachloride</a> <img src="../scipics/_wiki.png" /> or
fluorocarbons, where it forms a blue solution. At 161 K (−112 °C; −170 °F), it condenses to form a dark blue liquid. It is dangerous to
allow this liquid to warm to its boiling point, because both concentrated gaseous ozone and liquid ozone can detonate. At temperatures below 80 K
(−193.2 °C; −315.7 °F), it forms a violet-black solid.<sup>[8]</sup>
…
<strong>References</strong><hr />…<ol type="1" start="8"><li>^ <a
href="http://www.webelements.com/oxygen/" target="_blank">"Oxygen"</a> <img src="../scipics/_ext.png" />.
<em>WebElements</em>. Retrieved 2006-09-23.</li></ol> |
</a>
[Edited on 7/9/13 by bfesser]
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
some solubility data for O3 and O2
http://www.nist.gov/data/PDFfiles/jpcrd219.pdf
ozone in water
http://www.ozonesolutions.com/info/ozone-solubility
some more from google
http://books.google.com/books?id=PnjVAAAAMAAJ&pg=PA897&a...
this one is worth requesting I think
http://link.springer.com/content/pdf/10.1007%2F978-94-015-99...
hopefully helpful
-Violet Sin-
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
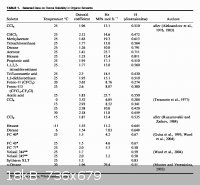
The above is from here _
Ozone Solubility in Liquids
www.sciencemadness.org/talk/viewthread.php?tid=9319&page...
( this is in the references section you require access to download )
Citation _
International Union of Pure and Applied Chemistry ( IUPAC )
Oxygen and Ozone ( Solubility Data Series; Vol 7 )
Battino , Rubin
Pergamon Press Inc. 1981
ISBN 0080239153
OCLC 7732154
Abstract: Evaluated literature data for solubility of oxygen and ozone in pure
liquids, mixtures, aqueous and organic solutions, biological fluids, etc. Focus
on gas/liquid systems and high and low pressures. Literature coverage to 1980.
System and registry number indexes.
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
From what I have read, ozone is soluble in carbon tetrachloride.
I am not really sure how compatible/stable this solvent is. Let's not forget ozone can oxidize chlorine dioxide, although this probably much due to
ClO2 being a radical molecule to begin with.
In fact, I think ozone is several times more soluble in CCl4 than water.
similar effect to dissolving O2 in perfluorocarbons
Oxycyte (perflourinated-tert-butylcyclohexane) is used as a blood substitute for carrying O2
the reason O2 is so soluble in it is because the intermolecular forces in the liquid are so weak
However, possibly unsafe to dissolve O3 in perfluorocarbons (other than CF4), it could potentially lead to explosive mixtures. (not sure about this
though)
[Edited on 10-7-2013 by AndersHoveland]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by testimento  | What I've read, ozone should be soluble in tetrachloride and sulfuric acid, but the solubilities are still rather limited, speaking of few grams per
liter maximum. Yes, it can be used as an oxidant, and according to my info, it will generate NH4NO3 from NH3(+H2O?), make SO3 of SO2, create lead
oxide in situ and corrode several materials.
Im planning on building electrolytic ozone cell, similar to chlor-alkali, but using only pure water with 0.5-1% NaOH as electrolyte: it should
generate up to 30% O3 with O2 at cathode and H2 from anode. |
How do you generate O3 instead of O2 at cathode?
On topic, I don't think it would be a good idea to mix strong oxidizer with chlorinated solvents, we are stabilizing them with ethanol all the time so
it doesn't form phosgene on reaction with air let alone ozone.
|
|
|
sargent1015
Hazard to Others
  
Posts: 315
Registered: 30-4-2012
Location: WI
Member Is Offline
Mood: Relaxed
|
|
Ozone is, in fact, blue
I am taking no credit for this, but Kristof has a picture on his blog of ozone in liquid nitrogen.
Ozone
Also, there's a picture of his arm showing the dangers of ozone 
(I am shamelessly promoting your blog today, I should get a bonus Kristof.  Also, your silver macro image is now my computers wallpaper!)
Also, your silver macro image is now my computers wallpaper!)
|
|
|