tsukasa
Harmless

Posts: 7
Registered: 14-8-2012
Location: Moscow, Russia
Member Is Offline
Mood: No Mood
|
|
Chloropicrin synthesis
Disclaimer: this is my first post on this forum, so fell free to correct me, if something is wrong with my post. Also, I'm not a native English
speaker, so my grammar is not perfect.
Introduction
According to [1], there are several possible routes of chloropicrin (nitrotrichloromethane, CCl3NO2, military code: PS)
synthesis, including chlorination of nitromethane using sodium hypochlorite, chlorination of picric acid, and nitration of chloroform.
The first route seems to be the most suitable for a small home lab. A quick search for detailed description of this procedure didn’t bring any
useful information, so I decided to experiment from scratch.
Later I purchased a book [2], where almost the same reaction conditions are described (but yield is not specified).
Precautions
Chloropicrin is toxic, it is a strong lachrymator, and in high concentration it causes pulmonary edema (which can be fatal). Liquid chloropicrin
causes skin burns. All operations should be performed in protective gloves. Operations, which may lead to contact with chloropicrin vapors should be
performed in gas mask.
Starting materials
The starting materials for this synthesis were reagent-grade nitromethane and technical grade sodium hypochlorite solution. This solution had been
stored for quite a long period, so we should expect significant loss of active chlorine. The solution’s density was measured: 1,23 g/mL. This
corresponds to 30 % concentration, and approx. 5 mols of hypochlorite per liter.
Hydrochloric acid and anhydrous calcium chloride (both reagent grade) were used during work-up.
General Procedure
Several runs were performed, so the exact values (amounts of reactants, reaction time, etc) will be listed later.
A 2 L flat-bottom flask was put on magnetic stirrer, it was equipped with pressure-equalized addition funnel and a reflux condenser. The condenser was
stoppered with a rubber stopper with a glass tube in it. The glass tube was connected to a hose and it’s other end was connected to empty Drexel
bottle following a Drexel bottle filled with a solution of sodium sulfide in ethanol/water (70 % ethanol v/w). This solution effectively adsorbs
chloropicrin vapors [1].
Hypochlorite solution was loaded into flask and nitromethane was loaded into addition funnel. Stirring was started (at RT) and nitromethane was slowly
added to flask. Reaction is slightly exothermic. No additional cooling or heating was applied. During the reaction, RM changes it’s color from green
to orange or red. After finishing of nitromethane addition, stirring was continued for some more time and then stopped. Layers separated (chloropicrin
forms a bottom layer). Upper layer was partially decanted, the residue was poured into measuring graduate (to find out the yield) and then into glass
bottle.
Workup. After completing all 5 runs, products were combined, separated from water layer in a separatory funnel, washed with some diluted hydrochloric
acid, dried over CaCl2 and decanted off desiccant.
Numeric data
- Run 1: 1.5 L NaOCl solution (7.5 mol, 3.3x excess), 40 ml (750 mmol) MeNO2 added over course of 2 min. Total reaction time: 30 min.
Yield: 40 mL (400 mmol, 54 %)
- Run 2: 1.5 L NaOCl solution (7.5 mol, 2.4x excess), 55ml (1.03 mol) MeNO2 added over course of 30 sec. Total reaction time: 30 min.
Yield: 40 mL (400 mmol, 34 %)
- Run 3: 1.7 L NaOCl solution (8.5 mol, 3.0x excess), 50 ml (935 mmol) MeNO2 added over course of 12 min. Total reaction time: 35 min.
Yield: 45 mL (450 mmol, 48 %)
- Run 4: 1.7 L NaOCl solution (8.5 mol, 3.8x excess), 40 ml (750 mmol) MeNO2 added over course of 10 min. Total reaction time: 40 min.
Yield: 48 mL (480 mmol, 64 %)
- Run 5: 1.7 L NaOCl solution (8.5 mol, 3.4x excess), 45 ml (840 mmol) MeNO2 added over course of 10 min. Total reaction time: 35 min.
Yield: 47 mL (470 mmol, 56 %)
Total: 8.1 L NaOCl solution (40.5 mol), 230 ml (4.3 mol) MeNO2. Yield: 215 mL (2,15 mol, 50 %).
Notes
Additional purification can be performed. It is possible to distill chloropicrin even at atmospheric pressure (though some decomposition may occur
[1]). Vacuum distillation is recommended [2].
Conclusion, plans for future experiments
The above procedure is very easy to perform, and has reasonable yield.
My future plans related to chloropicrin:
1. Try to use fresh (more concentrated) NaOCl solution
2. Use technical grade nitromethane (at last, it's available and about 4 times cheaper), this will allow to scale up a bit.
3. Perform vacuum distillation of product. This is quite difficult for me, because accidental spill of chloropicrin (there is always a risk of flask
explosion) will be a disaster for me and my neighbors.
4. Analyze the product to find out content of mono- and dichlorinated compounds. Some guys can even help me with NMR spectrum.
5. Write an article about refilling pepper sprays with chloropicrin. Some good results have already been achieved with Russian "UDAR" "impulse"
sprays.
6. Use chloropicrin for dichloroformaldoxime (phosgene oxime) synthesis. Presumably, I will use reduction with tin powder.
7. Synthesize chloropicrin analogue: 1,2-dinitro-1,1,2,2,-tetrachloroethane. This synthesis is quite challenging: it requires nitration of
tetrachloroethylene vapor with nitrous oxide. Reaction requires high pressure and temperature (though, I haven't studied it thoroughly).
References
1. Siegfried Franke. Manual of Military Chemistry: Chemistry of chemical warfare agents. U.S. Department of Commerce, 1968
2. Jared B. Ledgard. A Laboratory History of Chemical Warfare Agents. Second edition. Lulu.com, 2007, ISBN 0615136451, 9780615136455
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Very good.
You should hovewer know that sodium hypochlorite is usualy sold as 5 and 10%, because it is quite unstable and as such 30% is almost impossible. Take
note that hypochlorite solution contain a large amount of sodium chloride and sodium hydroxide, that would have greatly altered your results.
People love pictures.
[Edited on 24-6-2013 by plante1999]
I never asked for this.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by tsukasa  |
Starting materials
The starting materials for this synthesis were reagent-grade nitromethane and technical grade sodium hypochlorite solution. This solution had been
stored for quite a long period, so we should expect significant loss of active chlorine. The solution’s density was measured: 1,23 g/mL. This
corresponds to 30 % concentration, and approx. 5 mols of hypochlorite per liter.
|
My experience with degraded NaOCl solutions was that the density stayed the same even though the solution was degraded. For a true indication of
strength I recommend titrating with sodium thiosulfate solution.
That seems like a ballsy move to prepare a wargas on a multi-gram scale on 1st try. Why do you need so much of it?
[Edited on 24-6-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Too non-directional of a weapon to suit me. I would be afraid of collateral damage with something like this. You could even take yourself out if you
were to try to use it!
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Interesting write-up, but I dislike the pepperspray part. I also think that you made VERY much of this nasty stuff. A few ml or so would be nice, just
for some immediate experiments. I would not keep the stuff around for an unspecified amount of time. Use it up in some follow-up experiments.
As mentioned already by plante1999, the 30% bleach is not correct. I would say 15% at most, 10 to 13% is more realistic. The percentage is specified
as "available chlorine" and not as weight percent sodium hypochlorite. Please use the search engine to find more about this. Household bleach has
around 5% available chlorine.
I myself have considered doing this experiment on a micro scale as well, but the nasty properties of this compound and the strong smell, which is said
to stay around for a longer time have kept me away from this till now.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I suspect that making chloropicrin on the scale that the original author is doing is illegal.
Certainly loading it into pepper sprays is illegal in almost all places.
Discharging a spray like this at another person would almost certainly be classified as assault.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Relax, guys, he's from Moscow. I'm sure it's just for self defense:
<img
src="http://2-ps.googleusercontent.com/h/www.theleek.com/wp-content/uploads/2013/02/527x689xputin-riding-bear.jpg.pagespeed.ic.9V-VGW6i-q.webp"
width="350" />
|
|
|
neptunium
National Hazard
   
Posts: 990
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
what about the nitration of chloroform route? has anyone try this?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Chloropicrin is often added in very small quantities, dispersed in gas, during fumigations of houses. These fumigations typically use SO2F2, which is
odorless (due to its very slow rate of hydrolysis), the concept is to add a lachrymator to give anyone who may still be in the house warning.
Chlorination with hypochlorite of nitromethane proceeds through its aci-form tautomer. CH2=NO2‒
Without the elevated pH (since solutions of hypochlorite are moderately alkaline) the chlorination could not proceed.
Usually it is very difficult to nitrate alkanes, but the C-H bond in chloroform is very polar, because of those 3 chlorine atoms pulling away charge
distribution. (another example of this is the condensation of chloroform with acetone to form chlorobutanol)
Quote: Originally posted by tsukasa  | | 7. Synthesize chloropicrin analogue: 1,2-dinitro-1,1,2,2,-tetrachloroethane. This synthesis is quite challenging |
I wonder if 1,2-dinitroethane could be chlorinated with hypochlorite. Would the carbon-carbon bond be severed?
1,2-dinitroethane can be prepared in very high yield from reaction of NO2 with ethylene, in the absence of air. Alternatively, it could be done with
propylene, available in small welding gas cylinders.
No, just in certain places/countries – the type of countries that think making dangerous things illegal, even if they are homemade, can
somehow prevent crime. At first, I was a little perplexed and even annoyed at your comment. Then I realized you live in the UK, so I guess I should
not be surprised.
Still, does not sound like a very safe idea. What if it accidentally ruptures while it is being filled?!
[Edited on 24-6-2013 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I do not live in the UK.
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
He lives in Bretagne. But the way of thinking about this kind of things is not very different over there. And I agree with ScienceSquirrel that this
is illegal. The idea of putting chloropicrin in a pepperspray pressurized cylinder is a VERY bad one in my eyes, and using this thing is even worse!
|
|
|
tsukasa
Harmless

Posts: 7
Registered: 14-8-2012
Location: Moscow, Russia
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  |
Chlorination with hypochlorite of nitromethane proceeds through its aci-form tautomer. CH2=NO2‒
Without the elevated pH (since solutions of hypochlorite are moderately alkaline) the chlorination could not proceed.
|
Technical grade hypochlorite solutiuon contains 10-20 grams of NaOH per L (type A, according to our national standard) or 40-60 grams (type B) and
170-190 grams of active chlorine per L. My canister didn't have any label at all, unfortunately.
Great thanks to everyone for telling me, that this does not show the actual NaOCl concentration and an advice about titration. I think, I should
repeat the experiment to get more accurate data (and, of course, I will start it with titration).
I asked some guys about this reaction, some information (search results in Rexys) is attached.
Attachment: Chloroform --(HNO3)-- Trichloro(nitro)methane [Reaxys, All Preparations, 2013-05-08].pdf (68kB)
This file has been downloaded 1427 times
P.S. Of course the main purposes of my synthesis are:
1. Try to make some self-defense device, more efficient than commercially available ones, but still safe enough for use.
2. Use chloropicrin for further syntheses
3. Share it with one of my friends, who is also interested in this substance (without trying to make money on it, of course)
P.P.S. Several runs were performed not only for getting more product, but in the first place for finding out optimal conditions for reaction.
[Edited on 25-6-2013 by tsukasa]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I had been wondering whether there is a practical way to prepare nitromethane from the chloropicrin route.
I found a reference that states dinitrofluorochloromethane, ClFC(NO2)2, can be reduced with potassium iodide to dinitrofluoromethane. Not sure if this
is relevant. I have a feeling chloropicrin may not be a powerful enough oxidizer by itself. In any case, these types of compounds are very toxic and
dangerous. If you reduce the nitro group, it will result in phosgene oxime and you will likely kill yourself.
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Strong first post.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Since the compound is hazardous by itself, I'm wondering if reacting chlorine with nitromethane [1] rather than reaction an alkali hypochlorite with
it would be more or less of an hassle. I'm curious about which conditions are needed or if it goes as simple as bubbling chlorine in nitromethane.
[1] "The War Gases" by M. Sartori, which in turn refers to Kekulé, Justus Liebigs Annalen der Chemie, 1857, Volume 101, Issue 2, page 204.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I am fairly certain the chlorination here requires alkaline conditions, to convert the nitromethane into its aci- form tautomer.
(though if there is nothing present to chlorinate it, the aci- form is not very stable in the presence of water and degrades)
Under acidic conditions, nitromethane can be used as a solvent for concentrated nitric acid, apparently without reaction. (I have seen nitromethane
specified as the solvent in some nitration reactions using white fuming nitric acid)
There is also the reaction of nitromethane with aqueous mineral acid to form the hydroxylamine salt and formic acid, but I think this reaction
requires heating, please correct me if this is wrong.
| Quote: |
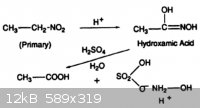
The action of strong aqueous acid on the primary nitroparaffins yields hydroxylamine as the acid salt, along with a carboxylic acid by-product (Fig.
8). The reaction proceeds through a hydroxamic acid intermediate.
"Speciality Chemicals: Innovations in Industrial Synthesis and Applications", edited by B. Pearson, p121 |
| Quote: | in a 500ml flask was placed 125 ml of nitromethane. this is refluxed and stirred. when solution is refluxing 125ml of sulfuric acid (30% conc.) is
slowly dripped into the solution over several hours. addition of sulfuric acid should take about 1 to 2 hours. entire reaction should take 5 hours
from time drip starts. after 5 hours, add mixture to equal weight alcohol and leave to cool. filter out quantitative yield of hydroxylamine from
nitromethane. reaction may also release some carbon monoxide so use adequate ventilation
|
I think this reaction is one of the ones which bears the name of Victor Meyer, if you want to do more research into the subject (Victor Meyer, Ann.
(1876) p.663). The Nef Reaction (John Ulric Nef) is similar, using mineral acid to convert nitroethane into acetaldehyde and nitrous oxide.
Apparently, the reaction can go either way, depending on reaction conditions, though I am not sure what exactly these conditions are. (obviously it
does not matter whether it is nitromethane or nitroethane)
[Edited on 13-7-2013 by AndersHoveland]
|
|
|
I Like Dots
Hazard to Self
 
Posts: 69
Registered: 10-4-2013
Member Is Offline
Mood: frisky
|
|
I did this today with nitromethane and 10% sodium hypochlorite.
To a seperatory funnel 1ml of nitromethane and 75ml of NaOCl were added. NaOCl was added first.
The mixture was gently swirled and became cloudy within a minute, and after 10 minutes the reaction appeared complete.
a small amount of orange/red liquid had settled to the bottom . I decided not to open the funnel, but instead used a small pipette to withdraw all
.2ml of this liquid.
While cleaning my glass pipette I sucked up water, and squirted it down the drain. I was immediately greeted by a strong irritation in one eye similar
to the sting of soap. Obviously when I was rinsing the pipette I atomized a small amount of chloropicrin.
An hour later, .5ml of clear liquid had settled on the bottom of the funnel. What is this liquid? I know chloropicrin should be clear. It must have
been Chloropicrin or possibly chloroform?. I was to gunshy to collect it again so I trashed it.
|
|
|
Baffled
Harmless

Posts: 16
Registered: 24-1-2014
Location: Milky way
Member Is Offline
Mood: No Mood
|
|
Seems like chloropicrin is on the rise as a pesticide, reapproved in 2008 it says on the wiki.
http://onlinelibrary.wiley.com/doi/10.1897/04-614Ra.1/pdf
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
May I suggest NH3 to dispose of chloropicrin, yielding harmless guanidine? 
|
|
|