DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
Help for the battle of the batteries
Hello people!
Since I'm quite new on the forum i am not sure or i post this in the right area.
It is school related, so I posted over here.
We're getting introduced to redox chemistry on my school. In relation with that they organized a battle. The battle of the batteries.
The goal is to make the best battery in a few hour’s with some given equipment and chemicals.
With “best” they mean the battery with the highest voltage and/or the most creative use of materials.
We are allowed to use the following equipment.
-Beakers (Not larger than 100ml)
-3M KCl-solution
-Paper-towels (Salt bridge)
- Graphite electrodes
-CuSO4*5H2O (s) (max 10,0000 gram)
- ZnSO4 (s) (max 10,0000gram)
- 1M acetic acid (max 50,00 ml)
- Aluminum Foil
- Copper plates
- Zinc plates
- demineralized water
- 0,02M KMnO4-solution(max 15,00 ml)
The are some simple rules:
-The maximum concentration of the solutions you use is 1M
-External power supplies aren’t allowed (duh)
My teacher told me that I was allowed to cheat ( don’t ask me why). He told me that if I want so, I can use materials from home. So if I find
something which can help me with the battery it would be great.
I would be very cool if you guys are able to help me with designing a battery to build.
I had the concept which I posted in the attachment in mind.
Do you guys think that this is a good method for reaching high voltage? Or do you have other ideas to obtain a higher voltage.
Thanks!
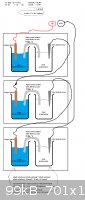
[Edited on 2-4-2013 by DutchChemistryBox]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
An aluminum anode (beer-can metal, scrubbed of it's coating works well) with any cathode (Cu, C, etc.) in a solution of household bleach (cut 1:2) and
a sprinkle of KMnO4 delivers useful voltage capable of delivering >100 mA/cm. If the alloy contains Mn (IIRC beer-can metal is an alloy that does),
the KMnO4 is not needed. When the cell is exhausted, the liquid turns pink--from the oxidized Mn leached from the metal. Refreshing the liquor in the
cell (so long as Al remains) perks it back up.
You also get a significant amount of H2. This could be put through a fuel cell (conceptually) for increased overall efficiency.
Note that the energy you get out was put in by the Al production process. No free lunch.
Cheers,
O3
[Edited on 2-4-2013 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Brominator
Harmless

Posts: 14
Registered: 21-2-2012
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
In an electrochemical cell the voltage is determined by the standard electrode potential of the chemicals in the cell so by choosing sutible chemicals
you can create a cell with a higher voltage. e.g a cell with set up
[Li][Li+][cr2o72-][Pt] will produce a voltage of 4.37V but would have to be set up in a sutable solvent due to the lithium. so the voltage you can
achieve all depends on the chemicals you have to hand.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
see:
http://www.youtube.com/watch?v=_FxIzMwOF00
http://www.youtube.com/watch?v=OcGWbt1mcrc
also see plante1999's website, i believe he has something on this topic.
all above information is intellectual property of Pyro.  |
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
You might find this helpful for selecting your chemistry:
http://en.wikipedia.org/wiki/Standard_electrode_potential_(d...
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
Thanks for the reactions!
Today I was in the lab for some tests, so I quickly build the battery which I had in mind. (The setup from the picture in the post earlier).
I reached a voltage of 2.8v . I'll attach a picture of it.
@Ozone
In your example you use aluminium as anode, so the aluminium will lose his electrons to the copper.
I do not understand the role of KMnO4 in the reaction. Is this a catalyst?
Could I reach a higher voltage with adding some KMnO4 solution to my setup?
@Brominator
It would be great if I could make a cel with something like lithium. But we don't have lithium on my school and I neither have it at home.
So I guess I am forced to use common materials.

|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
I think that I am going to make te battery with magnesium and copper.
V=V(copper) - V(magnesium) = 0.34 - -2.73= 3.07volts!
Copper plate in 0.5M CuSO4
and a Magnesium Pencil Sharpener in 0.5M Mg(NO3)2
As salt bridge I want to use a paper towel soaked in KCl
If I make three of those cells and switch them in series I have a cell of 3*3.07=9.21volts
Is this a good idea? Or is this somehow not possible?
Thanks
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
You could try different concentrations (as in 1 M CuSO4 and no Mg(NO3)2) to get a higher voltage:
http://en.wikipedia.org/wiki/Nernst_equation
Of course, the Nernst equation is not accurate at low concentrations, but having no Mg++ ions will raise the cell potential. Make sure to have some
other solute to increase the conductivity.
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
We use 3M KCl as salt bridge. Is it an option to use the same solution for the half-cell with the Mg inside?
What is the reason that a lot of examples on the internet are containing the iones of the kathode (not sure or I am saying it right in english).
For example the daniell cell, it contains Zn2+ in the solution of the anode, is this for recharging?
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
You will need an anhydrous polar solvent that Mg is inert in and dissolves the Mg salt of choice. Like Al, Mg passivates in air and water.
[Edited on 5-4-2013 by froot]
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
The Al:Mn system is non-passivating. It appears that the Mn operates catalytically and is involved with electron shuttling not only between the
anode:cathode pair, but with the reactive electrolyte, as well.
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Glucose Oxidase
Harmless

Posts: 37
Registered: 31-12-2012
Member Is Offline
Mood: Researching Alchemy
|
|
Make a silver zinc battery and i assure you , you WILL win  
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
Thank you guys for the information!
Now I'm ready to battle
@Glucose Oxidase
Bad enough that my girlfriend doesn't want to give me her silver rings for my battery
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
The battle was today, I did something totally different.
I've made a lot of mini galvanic cells in test tubes and putted them in serie.
With a result of 47.4volt!
I'm pretty happy with the result
 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4334
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Good job! Did you win?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
That's pretty darn good! Did you win?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
DutchChemistryBox
Hazard to Self
 
Posts: 74
Registered: 24-3-2013
Location: Strasbourg
Member Is Offline
Mood: No Mood
|
|
No unfortunately not  . .
The most of the teams didn't get any higher than 10 volt.
But one team won of us with a difference of 1 volt. There voltage was a little higher than 48 volt. They made an extremely high voltaic pile.
|
|
|