Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Ideas for a solvent trap/scrubber?
I've just bought a new vacuum pump on the net and don't want to destroy it by pumping through solvent vapours, potentially some acidic vapours and
more. The ideal solution, of course, would be LN2 trap with a base scrubber, but a) I don't have that kind of equipment, and b) have no access to LN2
or even dry ice.
Any ideas? I first thought of using a Dreschel bottle filled with NaOH or similar and submerged in an ice/salt mixture. However, I don't have one of
these, and, on eBay, the bottle and head will cost me £30, money I don't really want to spend if avoidable.
If it is of any significance, the innards of the pump are constructed from 'wetted aluminium'....
(see page 2, model no. 72R655-V10-C222TX
http://www.wainbee.com/gast/roc-r/71r72r_twin.pdf )
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
TheChemINC
Hazard to Self
 
Posts: 61
Registered: 22-2-2012
Member Is Offline
Mood: fuming
|
|
maybe even ice and salt could work? just make a trap that uses an aluminum or copper pipe that is inbetween your apparatus and pump.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Are you suggesting that we use copper/aluminium pipe as the trap itself?
I think the idea of a trap is that the vapours are condensed, and collected where they couldn't accidentally be re-sucked back into the pump, perhaps
even as a liquid/mist, which could be even more harmful to the pump.
I'm sure that ice and salt will be cool enough, I'm more concerned about the vessel itself to house the trap.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
TheChemINC
Hazard to Self
 
Posts: 61
Registered: 22-2-2012
Member Is Offline
Mood: fuming
|
|
no, just because it conducts better. but actually it might be better to just run it into a tube that is sitting in the ice and salt mix. just have the
tube that comes from the apparatus go to the bottom of the tube and then one at the top that leads to the pump. it would act like a condenser and by
releasing the vapors at the bottom, there would be more time for them to condense
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
You've basically just described a Dreschel bottle 
The "ad-hoc test tube idea" should work in theory, but it's not quite what I'm after, TBH - it's not a perfect solution. Besides, I have no stoppers
or anything with two holes in it for this, nor any glass tubing.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
TheChemINC
Hazard to Self
 
Posts: 61
Registered: 22-2-2012
Member Is Offline
Mood: fuming
|
|
hmmmmmm...........
what about using plain old 1/4" copper tubing? like the king that is used for the water connection to your refrigerator? that should work, and then if
you can, go to the hardware store and you can usually buy stoppers there. jsut find one that fits your tube/flask and bore two holes in it.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
You can DIY a gas-wash bottle really easily... Just get a nice big fat 2L pickle jar with a plastic lid, and cut 2 holes in it for the inlet/outlet.
Use silicon gel to seal around the rubber hoses going in and out. You should be able to make it with stuff you have lying around the house.
Here is a 5-minute paint image to give you an idea of what I mean:
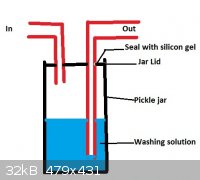
|
|
|
TheChemINC
Hazard to Self
 
Posts: 61
Registered: 22-2-2012
Member Is Offline
Mood: fuming
|
|
i think you have mixed up the in and out tubes. in this setup, the washing solution would be carried up the out tube. just switch them 
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I just bought a second trap off ebay. The first grabs volitiles the second oxygen. You should also have a water trap made of drierite or zeoliotes
or both. I'll copy and edit-add a diagram of the setup. Congratulations! Look for a manostat and manometer too. I have an extra Mcleod I might
part with.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Is there not a risk of suckback into the collection flask with this method, though?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Yes, there would... So you'd need a second one to avoid that.
Anyways, I would never ever pull a vacuum on a fucking pickle jar filled with a corrosive scrubbing solution. Amateur science is all fine and dandy,
but half-assing stuff like this is what leads to accidents!
Get a used dewar condenser on eBay, and fill it up with a salt/ice mixture. Add a tube with a dessicant for added protection.
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
There are a couple downsides to what you suggest Lambda; A salt ice mixture reaches at best about -20*C. How long is it going to stay at that
temperature though? Also, you've made no provision for scrubbing corrosives (ammonia, HCl).
I second what you say about the pickle jar. If you can avoid it (or unless the piece is designed to go under vacuum), don't pull vacuum on a flat
bottomed container. I've always wondered if dreschel bottles are "vacuum safe" because of this. The glass insert for my refrigerated trap also has a
flat bottom, but clearly this would have been accounted for in the design and fabrication of the piece. I'm not sure if the same can be said of
dreschel bottles.
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Quote: Originally posted by DJF90  | | There are a couple downsides to what you suggest Lambda; A salt ice mixture reaches at best about -20*C. How long is it going to stay at that
temperature though? Also, you've made no provision for scrubbing corrosives (ammonia, HCl). |
About the ice/salt mixture - according to Wikipedia you can reach as low as -40 degrees C with a mixture of calcium chloride and ice (I think the ratios are weight based). I don't know how
long it will stay at that temperature, but I've been thinking of doing some experiments on just that, because I'm skeptical myself, both of the
temperatures claimed and the duration. Obviously it will be important to crush the ice properly and mix it intimately with the salt of choice. Calcium
chloride is readily available and cheap as chips, so it would be interesting to see how much of an effect it would have. Dry ice is absolutely no
option for me and many others.
When I have some time and boredom to kill, I'll go ahead and do a series of experiments on it 
And about corrosives - my suggestion was just for general use where you want to protect the pump from water vapour and organics. In cases where you
also want to protect from corrosives it could be as easy as adding a drying tube with sodium or potassium hydroxide to deal with acid fumes and one
with sodium bisulfate to deal with bases. These would have to be in the line before the dessicant because the acid-base reactions would generate
water. I don't know how the hydroxides compare to other dessicants when it comes to sucking up water vapour, but maybe the dessicant could be skipped
altogether if you placed the hydroxide last in the line.
Quote: Originally posted by DJF90  | | I second what you say about the pickle jar. If you can avoid it (or unless the piece is designed to go under vacuum), don't pull vacuum on a flat
bottomed container. I've always wondered if dreschel bottles are "vacuum safe" because of this. The glass insert for my refrigerated trap also has a
flat bottom, but clearly this would have been accounted for in the design and fabrication of the piece. I'm not sure if the same can be said of
dreschel bottles. |
I don't know about normal dreschel bottles, but these ones from Duran are IIRC constructed to handle 1,5 bar of pressure (or 0,5 bar of overpressure).
This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I guess you could always change the contents of the scrubber depending on whether you anticipate to be producing acidic/basic vapours, or even create
mixtures for these purposes e.g. one scrubber for basic (e.g. ammonia) vapours that are damp, such as a NaHSO4/CaCl2 blend.
Liquid scrubbers may also be possible, but once would have to be careful to avoid suckback.
I like the look of those, Lambda, but they seem a little out of my price range
https://uk.vwr.com/app/catalog/Product?article_number=201-03...
[Edited on 28-12-2012 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Skip using ice/salt mixtures. These can be unreliable over long lengths of time. Instead turn to dry ice which is increasingly becoming an item of
home commerce. There are at least 3 stores in my area selling it openly now and probably more that carry it but do not advertise. This should not be
an issue providing you do not need to run your pump every day. You can reliably capture every common solvent with a dry ice / acetone slush.
In my experience however solvent vapors will not wreck a pump but they will kill the oil which will cripple the efficiency of a pump only until the
oil is changed.
Not sure on the efficacy of it but in a pinch a trap of activated carbon may work to remove trace solvents from your vacuum stream.
Forget using a liquid trap unless your liquid is highly non-volitle (conc. H2SO4 for instance). In industry however solid KOH traps are common
(U-tubes) to catch corrosives before they hit the pump. If you had a KOH trap (which should last some time, months maybe years if you're not abusing
it) and a dry ice/acetone trap you will be running the same as most industrial setups,
Note however that your pump doesn't have a massive amount of suck, so these nuggets of wisdom may not apply. I usually work at <0.001 mmHg whereas
(if I am understanding the chat correctly) your pump will be pulling in the neighborhood of 51 mmHg
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
That's correct, it's just for general filtrations, some distillations and low-pressure work; nothing that requires high vacuum.
I used to have a ground glass U-tube that would have been ideal, but alas someone broke it last year
Here in the UK, there is nowhere near me that will sell dry ice to individuals, with the exception of one or two companies who charge ridiculous
prices for it.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Bump
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|