pyrofrench
Harmless

Posts: 2
Registered: 2-10-2002
Member Is Offline
Mood: No Mood
|
|
camphor peroxide
hello everyone i'm a french pyro.
i'am 19, i leave nearby to paris.
my question: is it possible to make camphor peroxide ?
acetone is a keton
MEK is a keton
and camphor as well so why not.
sorry, i speak not very well english !
thank you for your answer.
PS in french there is only one forum "pyro"
[Edited on 28-7-2004 by chemoleo]
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
I think that it is possible to make camphor peroxide. But I may be wrong as my chemistry isnt all that good.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
I've thought of it in the past, but when you look at the structure of camphor you'll see that the OB would suck, and I think the peroxide would be
even less stable than most, if preparable.
|
|
|
BLAST_X
Harmless

Posts: 29
Registered: 21-9-2002
Location: USA
Member Is Offline
Mood: No Mood
|
|
You should test to oxidize synthetic camphor to form a peroxide or a hydroperoxide.
bornane-2-one
1,7,7-trimethylbicyclo(2.2.1)heptan-2-one
C10H16O
all you need is C - H - O - N
|
|
|
Boob Raider
Harmless

Posts: 33
Registered: 15-10-2002
Location: Canada
Member Is Offline
Mood: Picros
|
|
I doubt its
going to work. Camphor ain't soluble in H2O. I don't think peroxidation works well in alcohol, and if you use acetone, you'll end up with AP. But as
of now an alcohol soln is worth a try. Although if you boil camphor in conc HNO3, you will probably get nitro camphor or something which you can again
try to peroxidize.
|
|
|
trillian
Harmless

Posts: 1
Registered: 23-10-2002
Location: on top of a HNO3 drop
Member Is Offline
Mood: No Mood
|
|
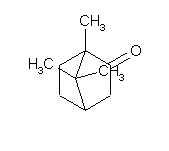
Camphor
Camphor are a very varied usable compound.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Does exist camphor peroxide?
Hallo,
I'm doing a research about the ketone camphor and I need to know if it can formate peroxides. If anyone can help me, saying yes or no and, if
yes, the equation.......this time Google haven't helped me.
Thanx 
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
terpene peroxides
If it is unsaturated (with a C=C double bond), a terpene should certainly be capable of forming a peroxide. Camphor (which contains a keto group) does
not meet this requirement, but camphene, which contains a C=CH2 group, does.
An example is cineole, a major constituent of eucalyptus oil, which is a naturally occurring terpene peroxide, and indeed probably largely responsible
for its antiseptic properties. I believe it, or a similar compound, occurs in teatree oil (either melaleuca (Australia) or leptospermum (New Zealand)
oil), which is also antiseptic. I remember reading somewhere that an industrial process for manufacturing hydrogen peroxide was, and may still be,
based upon the synthesis and reaction of a terpene peroxide.
John W.
(this was actually written by JohnWW but accidentally "reported" instead of "replied"
PGP Key and corresponding e-mail address
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The discussion on camphor peroxide indicated that it would suck as an explosive and that it would be difficult to make, but that doesn't rule it
out.
Acetone is a bit short on carbon-carbon double bonds, but I hear it forms a peroxide.
IIRC tea tree oil is largely terpineol; it might be possible to replace the OH with OOH but it wouldn't really be on- topic.
Last time I checked, the industrial production of hydrogen peroxide used the oxidation and reduction of a substituted anthraquinone.
|
|
|