1911
Harmless

Posts: 13
Registered: 29-6-2011
Location: Finland
Member Is Offline
Mood: No Mood
|
|
Selective methylation of phenol
I have a problem methylating a phenolic oxygen without touching the primary amine in the same molecule. Is there a way to do that. If I prepare a
phenoxide and react that with methyl iodide should the phenoxide group react faster than amine group or do I get a too many side reactions?
I tried googling 'selective methylation of phenols' but that didn't provide anything useful.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
One option would be oxidizing the amine to a nitro group first. Then simply distill the phenol with conc H2SO4 and methanol. Lastly, reduce the nitro
back to an amine.
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
You can also make the CBZ protected amine or if you're not using an acid, even the BOC protected amine. CBZ can be removed with mild catalytic
hydrogenation and BOC by stirring in relatively dilute HCl/MeOH or in trifluoroacetic acid.
Usually if you can selectively deprotonate the phenol, you can selectively methylate it if you add the methyl iodide slowly and not in excess.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Generally, the amine will be alkylated preferentially, even if you pre-form the phenoxide. As stated, you need to protect the amine. If you
show/explain the exact transformation you want to accomplish, maybe someone can give you a more specific answer
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
1911
Harmless

Posts: 13
Registered: 29-6-2011
Location: Finland
Member Is Offline
Mood: No Mood
|
|
Here is the desired reaction. Should the indolic nitrogen form another problem in the methylation step? I'm aware that there are other ways to
synthesize the target chemical but I'm trying to find a theoretical way from 5-hydroxytryptophan.
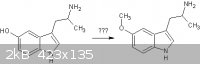
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Indole should be OK but the primary amine is toast. You need to protect! But doing that OTC is easier said than done.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
1911
Harmless

Posts: 13
Registered: 29-6-2011
Location: Finland
Member Is Offline
Mood: No Mood
|
|
I got an idea about using Kolbe electrolysis to decarboxylate the carboxylic group from 5-HTP and replace that with methyl group from acetate salt.
When the amino acid is deprotonated and excess of base is used, that will deprotonate the phenolic oxygen too? When Kolbe electrolysis is applied with
acetate salt in excess would the phenolic oxygen become methylated too from methyl radicals present?
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 1911  | | Here is the desired reaction. Should the indolic nitrogen form another problem in the methylation step? I'm aware that there are other ways to
synthesize the target chemical but I'm trying to find a theoretical way from 5-hydroxytryptophan. |
Well, good luck. I can't remember having seen a convincing phenylalanine to amphetamine route and tryptamines are more sensitive than phenethylamines.
Aiming high is laudable, but maybe chose a simpler target first?
|
|
|