Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Chloroform woes
Folks,
okay, this sounds like kinda stupid, since the haloform reaction is one of the most straightforward. Yet, somehow, I can’t get it right. It does
produce chloroform, but it is obviously mixed with something else, and no matter how much calcium chloride or even molecular sieves I mix in, I
can’t get the distillation to plateau at 60 °C, so it’s obviously not water (the liquid is very clear). The vapour temp just rises right from 58
°C to almost 85 °C with no real stop, and what I collect, even after two or three consecutive distillations, is highly impure.
Okay, I didn't use a fractional column, but I never assumed I'd have to. I’m not aware of any side reaction mixing acetone with bleach can produce,
and acetone boils at 56 °C, so that’s definitely not it either.
I tried to use that raw chloroform for a Riemer-Tiemann reaction to produce hydroxybenzaldehyde from phenol (see Vogel p. 997), but I got a miserable
yield despite using TBAB as a phase transfer catalyst (which Vogel doesn’t ask for), which I attribute to the poor quality of my chloroform.
Otherwise, that reaction is fine and quite interesting to try out.
Any idea or experience to share to improve the quality of a haloform reaction?
Thanks a bunch!
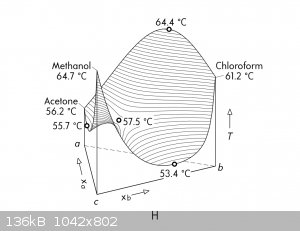
[EDIT: Actually, I just found out that acetone and chloroform form a positive azeotrope at 64 °C, which this ternary diagramme details.]
[Edited on 22-8-2023 by Keras]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yeah, it’s acetone. I found that to be the case some years ago when I checked my homemade chloroform by NMR. Repeatedly washing it with water (and
then re-drying it, of course) should bring down the amount of acetone present.
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Texium  | | Yeah, it’s acetone. I found that to be the case some years ago when I checked my homemade chloroform by NMR. Repeatedly washing it with water (and
then re-drying it, of course) should bring down the amount of acetone present. |
I’m still surprised the thermometer rises over 70/75 °C. I agree there is a real plateau at 64 °C, which I erroneously assumed to be a somewhat
defective thermometer accuracy. But 70/75 °C?
Isn't there any other means to get rid of the acetone? Because, well, I don’t want to rain on anyone's parade, but chloroform IS soluble in water to
some extent, and with repeated washing you can end up losing a lot of product.
Could it be a better idea to make chloroform using, say, methyl ethyl ketone (a.k.a butanone) instead?
[EDIT: No, there’s also a maximum azeotrope with butanone. However, apparently acetone and n-hexane form a minimum azeotrope that boils off at 49.8
°C. So, by adding enough n-hexane, one should be able to carry away all the acetone, leaving only pure chloroform. But of course, nothing is ideal in
this vale of tears, and chloroform and n-heptane also form a minimum azeotrope, which means you end up having to separate both…]
[EDIT EDIT: If it’s acetone, then it could be turned into more chloroform just by adding more bleach. Wouldn't that work?]
[Edited on 22-8-2023 by Keras]
[Edited on 22-8-2023 by Keras]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Keras  | | Isn't there any other means to get rid of the acetone? Because, well, I don’t want to rain on anyone's parade, but chloroform IS soluble in water to
some extent, and with repeated washing you can end up losing a lot of product. |
You would only lose a couple
grams that way, at most.
Quote: Originally posted by Keras  | | If it’s acetone, then it could be turned into more chloroform just by adding more bleach. Wouldn't that work? |
Just use a larger excess of hypochlorite next time you make it.
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Yeah, probably a good idea.
I’m going to use calcium hypochlorite I think, instead of 10% bleach. I can even recycle calcium acetate to get back acetone, probably with a
greater purity than the industrial one.
[Edited on 23-8-2023 by Keras]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Keras  | | I can even recycle calcium acetate to get back acetone, probably with a greater purity than the industrial one. |
Probably not. Industrial acetone is actually extremely pure, at least here in the US, though I would assume it's produced by the
same process globally. It's a conceptually interesting idea, but likely not worth it.
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Texium  | Quote: Originally posted by Keras  | | I can even recycle calcium acetate to get back acetone, probably with a greater purity than the industrial one. |
Probably not. Industrial acetone is actually extremely pure, at least here in the US, though I would assume it's produced by the
same process globally. It's a conceptually interesting idea, but likely not worth it. |
I tried industrial acetone to make dimethyl phenyl carbinol from my BnBr using Grignard. The preparation of the Grignard reagent was surprisingly easy
(the quality of the Mg chips was higher than I assumed), but as soon as I dropped the acetone, there appeared white flakes of magnesium hydroxide…

|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
How do you know it was magnesium hydroxide and wasn’t just the Mg alkoxide product being formed? Even if it was hydroxide, wet acetone is an easily
solved problem, and doesn’t prove that there are other more problematic impurities present.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If the only impurity in your acetone is water, and you are going to add it to bleach, there's no point drying it.
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Texium  | | How do you know it was magnesium hydroxide and wasn’t just the Mg alkoxide product being formed? Even if it was hydroxide, wet acetone is an easily
solved problem, and doesn’t prove that there are other more problematic impurities present. |
Oh, I don’t pretend there are other impurities present. Just that it'd be nice to have a batch of acetone free of water to begin with. Of course, I
could add molecular sieves or even calcium chloride to my industrial acetone!
|
|
|