SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Recrystallizing molten sulphur in a calcium chloride brine
In many commercial fertilizers containing elemental sulphur, the sulphur is fused with a large amount of dirt, necessitating further purification. The
typical methods cited on these forums are dissolution of the sulphur in hot, highly flammable aromatic solvents (such as toluene or xylene) or distillation of the sulphur at high temperatures in range of its 445°C boiling point.
These procedures can be difficult or even dangerous to attempt if you don't have the right equipment, so I looked into a safer, more accessible method
of purifying elemental sulphur which was eluded to in a late 19th century paper that I can no longer seem to find.
The TLDR is you can purify sulphur by melting it in a pot with some concentrated calcium chloride solution and collecting the beads of molten
sulphur once they've resolidified. A brief video is available here. I've got more to say though.

Sulphur purified from garden fertilizer by the method in this thread.
The melting of the sulphur is mediated by a calcium chloride brine with a concentration in excess of 35-40% CaCl₂ by mass, which is required to elevate the boiling point of water above 115-120°C, which is the temperature range quoted for the
melting point of the alpha and beta sulphur allotropes.
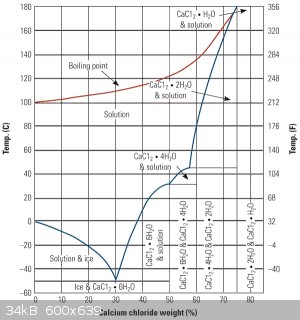
According to this phase diagram, the maximum boiling point of a calcium chloride brine could reach an astounding 175°C at a concentration of 78% CaCl₂ by
mass, beyond which it collapses to the dihydrate.
Aside from raising the boiling point of water, the brine serves several other purposes, which are that it:
1. lowers the exposure of the sulphur to air, which reduces fuming and prevents the sulphur from catching fire.
2. agitates the molten sulphur globs, separating them from any entrained dirt.
3. floats the sulphur above any impurities that are denser than the brine.
The density of molten sulphur is around 1.82 g/cm³, which is quite a lot lighter than silica and clay minerals (~2.65 g/cm³). I'm not sure what
concentration of calcium chloride is required to exceed the density of molten sulphur, but it's probably in excess of 70% CaCl₂ by mass.
  
A beaker of melting sulphur, a large red globule of molten sulphur floating atop the brine, and the same glob, crystallized as a shiny yellow
'patty'. Brief video available here.
The sulphur globs, which are surprisingly fluid, typically don't entrain the calcium chloride brine as they solidify due to their high cohesion, which
I'm guessing is due to the difference in molecular polarity between the polar brine and the non-polar orthorhombic octasulphur (S₈) molecules.
Nevertheless, the sulphur beads can be broken apart and washed clean to ensure their purity.
-
The offgassing of sulphur fumes is low enough that this process is safe to perform indoors with a few windows cracked open.
I suspect there may be other specialty applications for the calcium chloride brine regarding its extremely high density, boiling point and inertness
with respect to other common liquids.
Tags: Purifying molten sulphur in a saltwater brine, melting sulphur in saltwater, separating sulphur from dirt impurities
by melting, purifying sulphur by fusion in hot water
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Alternative brines
This is a speculative, untested list of alternatives to the calcium chloride brine, which get progressively worse and ill-advised.
A suitable substitute has to have a boiling point of at least ~120°C and ideally should be chemically inert and easy to
obtain. I think the best option would be to use some sort of pressure vessel (such as an ordinary pressure cooker) to elevate the boiling
point of pure water above 120°C, which would only require around 2 atmospheres of pressure. I believe this is by far the best alternative.
-
Aside from calcium chloride, magnesium chloride may also work, as well as magnesium acetate and sodium acetate. Phosphoric acid should work, as well
as the triphosphate salts (NaH₂PO₄, KH₂PO₄ or NH₄H₂PO₄).
Sugar (sucrose) might be a viable option, if a bit sticky, flammable and prone to decomposition. Concentrations in range of 90% sugar by mass may be required to hit 120°C. It's worth noting that if the sugar hydrolyzes (this typically occurs under acidic conditions)
you may have to be concerned about the production of hydrogen sulphide gas caused by reduction of sulphur via the aldehyde functional group in the
monosaccharides. This is very speculative.
-
If for some reason you're not concerned with using hot concentrated alkalis, potassium carbonate or hydroxide (the sodium analogs likely aren't
soluble enough) might work, although the latter may react with elemental sulphur to form potassium sulphides, sulphites and thiosulphates, perhaps by the following formulas:
6KOH(aq) + (5+n)S(l) -> K₂Sₙ(aq) + 2K₂SO₃(aq) + 3H₂S(aq/g)
6KOH(aq) + (7+n)S(l) -> K₂Sₙ(aq) + 2K₂S₂O₃(aq) + 3H₂S(aq/g)
I'm not familiar with alkaline sulphur chemistry, however.
-
Sulphuric acid can reach a boiling point 120°C at a surprisingly high concentration of at least 50%, though aside from being corrosive, I believe
sulphuric acid and elemental sulphur tend to redox to water and sulphur dioxide, like so:
2H₂SO₄(aq) + S(l) -> 2H₂O(l) + 3SO₂(g/aq)
I'm not sure what temperatures are required for this reaction to become a problem.
-
Vegetable oils can easily reach temperatures in range of 300°C and I believe should be fairly non-flammable at 120°C, but I don't recommend using
them because of the absolutely abysmal mess they make.
Aliphatic hydrocarbons such as kerosene and mineral spirits with a boiling point between 150-225°C are less greasy and will evaporate within a
reasonable amount of time if spilled, but are extremely highly flammable near their boiling point.
Additionally, I think that both vegetable oil and hydrocarbons have a slight tendency to dissolve elemental sulphur, and may react to form hydrogen sulphide gas:
CₙH₂ₙ₊₂(l) + S(l) -> CₙH₂ₙ(l) + H₂S(g)
-
Many (if not all) of the nitrate salts (NH₄NO₃, KNO₃, Ca(NO₃)₂, Cu(NO₃)₂ etc.) exhibit the solubility required to reach a boiling point
elevation well above 120°C, but are, of course, oxidizers, which have the potential to react with sulphur by some combination of the following
formulas:
2KNO₃(aq) + 2S(l) -> K₂SO₄(aq) + SO₂(g/aq) + N₂(g)
2KNO₃(aq) + 4S(l) -> K₂S(aq) + 3SO₂(g/aq) + N₂(g)
This could be a gradual reaction or it could be comparable to an explosion.
-
Similarly to the nitrates, a number of chromates and dichromate salts are soluble enough to effect a boiling point elevation of at least 120°C,
particularly the sodium, ammonium and magnesium di/chromates. However, besides being carcinogenic, they're also oxidizing agents which may react with
sulphur, possibly by the following formulas:
4Na₂CrO₄(aq) + 9S(l) -> 4Na₂S(aq) + 5SO₂(g/aq) + 2Cr₂O₃(s)
Na₂Cr₂O₇(aq) + 3S(l) -> Na₂S(aq) + 2SO₂(g/aq) + Cr₂O₃(s)
I'm not sure why you would have enough chromate salts to do this project, but what kind of scientist would I be if I didn't mention them.
Corrections and other suggestions are appreciated. If anyone wants to test these, I'd love to hear how it goes. Unless you have a good lawyer.
[Edited on 7/7/2023 by SnailsAttack]
|
|
|
BromicAcid
International Hazard
    
Posts: 3250
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Very cool, I like what you've done here. Nice looking sulfur.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Thanks. molten sulphur has such a beautiful
amber color, I wish it didn't fade as it crystallizes.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2739
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
That is a really cool experiment, never would have thought of using aq CaCl2 for that. But I have always been able to flours of sulfer in almost
any pharmacy, just in case you need a purer source of it, It should also be readily available from any pyro chemical source.
|
|
|
Texium
|
Thread Moved
15-7-2023 at 18:29 |
Sulaiman
International Hazard
    
Posts: 3704
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
A very interesting experiment.
The principles have generally useful potential
but in the case of sulphur
for as long as crude oil is refined
sulphur will be available in huge quantities
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | A very interesting experiment.
The principles have generally useful potential but in the case of sulphur
for as long as crude oil is refined[,] sulphur will be available in huge quantities |
Yeah. Even in absence of
Claus process sulphur from the oil industry, I think this method of refining sulphur is only of amateur interest.
Quote: Originally posted by Dr.Bob  | | That is a really cool experiment, never would have thought of using aq CaCl2 for that. But I have always been able to [find] flours of sulfer in
almost any pharmacy, just in case you need a purer source of it, It should also be readily available from any pyro chemical source.
|
Thanks. You probably can't beat flowers of sulphur as far as purity goes. I got a pound of fertilizer-grade
sulphur on sale at Lowes for like 3 bucks though, so I decided to refine it myself.
|
|
|
FrecherChemiker
Harmless

Posts: 3
Registered: 10-7-2023
Member Is Offline
Mood: Based
|
|
Nice work!
A pressure cooker would maybe be an alternative. At least you could reach the required temperature for melting sulfur without adding a salt.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by FrecherChemiker  | Nice work!
A pressure cooker would maybe be an alternative. At least you could reach the required temperature for melting sulfur without adding a salt.
|
Thank you. Yes, a pressure cooker would (in theory) work awesome for this, it takes forever to recycle the brine.
[Edited on 7/19/2023 by SnailsAttack]
|
|
|