SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Chromium chromate - does it exist?
Do chromium salts of di/chromate exist?
Chromium(II) chromate (CrCrO₄)
Chromium(III) chromate (Cr₂(CrO₄)₃)
I haven't heard anything about them, so I'm thinking maybe they decompose to oxides:
CrCrO₄ -> CrO + CrO₃
Cr₂(CrO₄)₃ -> 3CrO₃ + Cr₂O₃
If they do exist, I propose the umbrella name "Chromy McChromeface".
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Chromium(II) chromate definitely can’t exist since Cr(II) is a strong reducing agent while Cr(VI) is a strong oxidizer. You’d most likely see
comproportionation to Cr(III) species. I don’t see an obvious reason why chromium(III) chromate couldn’t exist, since iron(III) chromate is known,
but I tried making it before without any success. Granted, that was a long time ago.
|
|
|
Texium
|
Thread Moved
24-2-2023 at 07:09 |
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Texium  | | Chromium(II) chromate definitely can’t exist since Cr(II) is a strong reducing agent while Cr(VI) is a strong oxidizer. You’d most likely see
comproportionation to Cr(III) species. |
The formula for that was actually in the original draft of this post.
| Quote: | | 3CrCrO₄ -> 2Cr₂O₃ + 2CrO₃ |
Looks like you'd get equimolar quantities of chromia and chromic anhydride.
Quote: Originally posted by Texium  | | I don’t see an obvious reason why chromium(III) chromate couldn’t exist, since iron(III) chromate is known, but I tried making it before without
any success. Granted, that was a long time ago. |
Interesting. I'll try this sometime.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I'd expect that you'd just wind up with mostly CrO2.
2 H2O + 2 Cr3+ + CrO42- --> 3 CrO2 + 4 H+
And I know CrO2 exists, unless a decade's worth of cassette tapes were lying to me.
[Edited on 24-2-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Yes, they exist but mostly "chromium chromate/dichromates" are coordination compounds, like chromium chlorides and nitrates. Compounds with (NH3) and
(H2O) ligands could be easily prepared. I don't know whether it is possible to prepare anhydrous chromium
(di)chromates. Also, if I recall it properly H2O ligand in the case of (di)chromate cause polimerization ("olation") and the result is not so
well-defined as with NH3, mixed NH3 & H2O or ethylendiamine ligands.
Also I suspect some of them could be "energetic".
[Edited on 24-2-2023 by teodor]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I actually tried this a long time ago, by adding a solution of chromium(III) sulfate (or chrome alum) to a solution of potassium dichromate. If that
is done, then no precipitate is formed, the orange color of the dichromate and the dark greyish/purple color of the chromium(III) mix up to something
brown. On long standing, however, a very fine and compact dark brown precipitate is formed, which can be isolated (I decanted the solution, added
water, let it settle again, decanted again and repeated a few times). I think that this dark brown material is chromium(IV) oxide, CrO2.
With potassium chromate the result is much more unpredictable and strongly depends on concentrations of the reactants. I think that the solution is
too basic and leads to formation of ill-defined mixed/basic hydroxide/chromate compounds. I did not further investigate this. This solution is not so
strongly basic, that it completely dissolves the chromium(III) as green chromate(III).
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
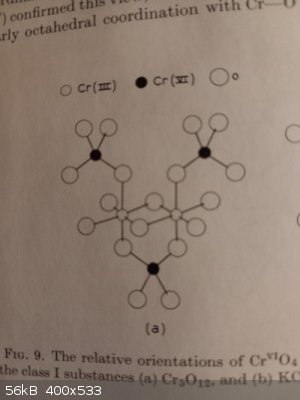
I found some information about "anhydrous chromium chromates" in the article "Mixed valency chemistry" in the "Advances in Inorganic chemistry and
radiochemistry" v.10 (1967). I remember there are full scans of this serie available in the internet, but I can do a scan of the "Chromium" part of
the article if you have difficulties to found it.
According to the article, the complexity of the structure of those compounds is caused by the fact that Cr(III) prefers octahedral coordination and
Cr(VI) - tetrahedral, so the structure is rather complex and all those mixed-valency compounds forms a lot of intermediates of which Cr2O5, Cr3O4,
Cr3O5, Cr3O8, Cr4O9, Cr5O9, Cr5O12, Cr5O13, Cr5O19, Cr6O15, Cr7O18, Cr8O15, Cr8O21 are known.
So, you can imagine all complexity involved with isolation and definition of the structure. But at least some of them are defined, like Cr5O12:
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by woelen  | | I actually tried this a long time ago, by adding a solution of chromium(III) sulfate (or chrome alum) to a solution of potassium dichromate. If that
is done, then no precipitate is formed, the orange color of the dichromate and the dark greyish/purple color of the chromium(III) mix up to something
brown. On long standing, however, a very fine and compact dark brown precipitate is formed, which can be isolated (I decanted the solution, added
water, let it settle again, decanted again and repeated a few times). I think that this dark brown material is chromium(IV) oxide, CrO2.
|
2Cr₂(SO₄)₃(aq) + 6K₂Cr₂O₇(aq) + 5H₂O(l) -> 6CrO₂(s) + 6K₂SO₄(aq) + 5H₂Cr₂O₇(aq)
Quote: Originally posted by woelen  | | With potassium chromate the result is much more unpredictable and strongly depends on concentrations of the reactants. I think that the solution is
too basic and leads to formation of ill-defined mixed/basic hydroxide/chromate compounds. I did not further investigate this. This solution is not so
strongly basic, that it completely dissolves the chromium(III) as green chromate(III). |
The formulas balance if the products are assumed to be chromium(III) oxyhydroxide and the weird mixed-valency polymeric oxides that teodor cited.
4Cr₂(SO₄)₃(aq) + 12K₂CrO₄(aq) + H₂O(l) -> 9Cr₂O₅(s) + 2CrO(OH)(s) + 12K₂SO₄(aq)
2Cr₂(SO₄)₃(aq) + 6K₂CrO₄(aq) + H₂O(l) -> Cr₈O₂₁(s) + 2CrO(OH)(s) + 6K₂SO₄(aq)
Differentiating Cr₂(CrO₄)₃ from CrO₂ or the mixed-valency oxides might be impossible.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Another route to the "weird mixed-valency polymeric oxides" could be aerial oxidation of Cr(OH)3*nH2O in highly alkaline environment, I posted once
this experiment but was failed to analyse how much Cr(III) was oxidised to Cr(VI).
I think working with cations like chromium always requires the cation to be stabilised by addition of proper ligands.
In the case of chromium ligands containing oxygen (H2O, OH) cause olation (I think it is more correct term than polymerisation, Bedlasky posted thread
about it) because O atoms tends to connect different Cr(III) kernels.
So, practically, if we would like to keep things as simple as possible, we can work well with ammonium ligands (also ammonium + water or ammonium +
halogen but for simplicity let's think first about hexamines).
From Cr(NH3)6(NO3)3 Jörgensen made [Cr(NH3)6]2(CrO4)2 and [Cr(NH3)6]2(Cr2O7)3 and published the procedure in 1884. Basically it is just reaction of
CrA6(NO3)3 with K2CrO4 or K2Cr2O7.
The more challenging is to get some chromium hexamine to start with.
Well-checked methods of preparation for CrA6(NO3)3 could be divided into 2 classes: those ones which use liquid NH3 and the second one - reacting of
CrA6Cl3 with HNO3.
To get CrA6Cl3 many routes could be used, I will not talk about it in this message.
I'd like to speculate a bit about liquid NH3 route which could be probably used in a home lab.
As you probably know, addition of NH4NO3 to liquid NH3 increases the boiling point. At some concentration it stays liquid at room temperature. And as
a consequence, this liquid (it has some special name, do you remember it?) could be prepared in a water ice bath (0C) by passing dry ammonia gas
through NH4NO3. It requires just cooling because the mixing is exotermic.
So, as a result you can get handy water-free room temperature liquid ammonium with some NO3- pollution but for preparation of Cr(NH3)6(NO3)3 it should
not be a problem (I think what you will get is just analogue of a nitric acid solution but in different solvent than water).
I believe this rote is either not explored or the result is not published or it was published somewhere but it is hard to find where exactly it was.
So, if you have inspiration for new experiments, let's try and keep everybody informed.
Just be careful, all complexes containing ammonia and oxidisers have explosive properties (typically, when dry) at some extend and first authors never
published a word about it. But I remember some species of CrO4- complexes (I am not sure it was CrA6) I found in the list of most sensitive comparing
to other anions (I could be wrong, but be careful).
[Edited on 25-2-2023 by teodor]
[Edited on 25-2-2023 by teodor]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by teodor  | I think working with cations like chromium always requires the cation to be stabilised by addition of proper ligands.
In the case of chromium ligands containing oxygen (H2O, OH) cause olation (I think it is more correct term than polymerisation, Bedlasky posted thread
about it) because O atoms tends to connect different Cr(III) kernels. |
You're right it is 'olation'.. I'll
take what chances I get to avoid polymer chemistry. The wikipedia page on olation has some good info.
Quote: Originally posted by teodor  | From Cr(NH3)6(NO3)3 Jörgensen made [Cr(NH3)6]2(CrO4)2 and [Cr(NH3)6]2(Cr2O7)3 and published the procedure in 1884. Basically it is just reaction of
CrA6(NO3)3 with K2CrO4 or K2Cr2O7.
The more challenging is to get some chromium hexamine to start with.
Well-checked methods of preparation for CrA6(NO3)3 could be divided into 2 classes: those ones which use liquid NH3 and the second one - reacting of
CrA6Cl3 with HNO3. |
I assume typical aqueous ammonia solution isn't enough to strip hexaquachromium(III) of
all its water and/or hydroxide ligands, yeah?
Quote: Originally posted by teodor  | | As you probably know, addition of NH4NO3 to liquid NH3 increases the boiling point. At some concentration it stays liquid at room temperature. And as
a consequence, this liquid (it has some special name, do you remember it?) |
Illegal everywhere but the US?
Jokes aside, that's wild, what is it called? I can't find any information about it online. I assume it'd be mostly ammonium nitrate since that'd
require a boiling point elevation of ~50 degrees.
Quote: Originally posted by teodor  | I believe this rote is either not explored or the result is not published or it was published somewhere but it is hard to find where exactly it was.
So, if you have inspiration for new experiments, let's try and keep everybody informed. |
Sure, I like
ammonia. Not sure I want to get into anhydrous chemistry just to say I made Chromy McChromeface, but I'll write this down.
Quote: Originally posted by teodor  | | Just be careful, all complexes containing ammonia and oxidisers have explosive properties (typically, when dry) at some extend and first authors never
published a word about it. But I remember some species of CrO4- complexes (I am not sure it was CrA6) I found in the list of most sensitive comparing
to other anions (I could be wrong, but be careful). |
Noted.
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  | Quote: Originally posted by teodor  | | As you probably know, addition of NH4NO3 to liquid NH3 increases the boiling point. At some concentration it stays liquid at room temperature. And as
a consequence, this liquid (it has some special name, do you remember it?) |
Illegal everywhere but the US?
Jokes aside, that's wild, what is it called? I can't find any information about it online. I assume it'd be mostly ammonium nitrate since that'd
require a boiling point elevation of ~50 degrees.
|
The name of the solution ammonium nitrate in liquid ammonia is "Diver's liquid". You can find about it in the Mellor's "A comprehensive treatise of
inorganic and analytical chemistry", Vol. II available in the Sciendmadness library. The information online is created by people like you and me. If
you expect to find it let's write something about it. To make it liquid at +12C you should mix 30g of NH3 and 100g NH4NO3. It is not "salt"
solution. It is HNO3 solution. This magic transformation (salt -> acid) happens because NH3 is the solvent.
And yes, there is probably a potential option to use water ammonia solution in some many-steps process to prepare hexamine . But to find something new
in my opinion it is better to make experiments with Diver's liquid.
[Edited on 25-2-2023 by teodor]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It’s not really too weird when you think about it this way: nitric acid in water is effectively an aqueous solution of “hydronium nitrate” since
it is a strong acid that fully dissociates (or in other words, protonates 100% of the water that it can), even though we don’t write it that way.
Replace the water with ammonia, and now you have ammonium nitrate dissolved in ammonia.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Chromium chromates or dioxide should routinely form when chromates are reduced. Because obviously if Cr(III) is formed, it forms something with the
excess chromate - whether chromium chromate or dioxide.
An obvious reagent to get clean chromium reduced forms is methanol.
CH3OH+3CrO3=CO2+3CrO2+2H2O
CH3OH+8CrO3=CO2+Cr2(Cr2O7)3+2H2O
No possible junk counterions. In the strong acid solution of excess H2Cr2O7, CO2 is poorly soluble and of the intermediates, CH2O does not ionize and
in strong acid neither does CH2O2. Both are rapidly oxidized.
When a concentrated clear CrO3 solution in water is slowly reduced by methanol, what do you get? Precipitate of black CrO2 while the orange H2Cr2O7
solution only eventually gets more dilute? Or does Cr(III) appear?
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
Quote: Originally posted by woelen  | I actually tried this a long time ago, by adding a solution of chromium(III) sulfate (or chrome alum) to a solution of potassium dichromate. If that
is done, then no precipitate is formed, the orange color of the dichromate and the dark greyish/purple color of the chromium(III) mix up to something
brown. On long standing, however, a very fine and compact dark brown precipitate is formed, which can be isolated (I decanted the solution, added
water, let it settle again, decanted again and repeated a few times). I think that this dark brown material is chromium(IV) oxide, CrO2.
|
Some sources say the precipitate of mixing Cr(III) and Cr(VI) in acidic solution has the variable stoichiometry H4Cr3O8 * xH2O and contains 2xCr(III)
+ 1xCr(VI) (determined with help of Cr(III) isotopes). When it lose water it becomes CrO2.
My sources are pre-1970, so I am not quite sure it is everything we can know about it.
[Edited on 26-2-2023 by teodor]
|
|
|