Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
ΔG Gibbs free energy
Attachment: Gibbs Free Energy Calculator.xlsx (73kB)
This file has been downloaded 285 times
Ramble:
I have been using "ΔG = ΔH - TΔS" to try to find new pathways to form reagents out of different materials and expand my skills a little.
Put together a spreadsheet to make it easier to look up the values, it will answer all the questions in my textbook on the subject within +/- 3
kJ/mol.
Not sure if that is good enough. I googled a list of thermodynamic values, not 100% confident in the accuracy of the information.
Currently, it only takes into account temperature. I might add pressure later.
It says water boils at 97c.
Hope that helps someone as its helped me understand chemical theory much better. For such an important part of chemistry, it's a very small chapter in
my book.
Questions:
Gibbs tells us if a reaction would be spontaneous under certain conditions
how do I calculate how fast a reaction should progress to equilibrium?
Can Gibbs be used to predict major/minor products? Example
H2 + ½O2 = H2O -228.58 kJ/mol
H2 + O2 = H2O2 -120.40 kJ/mol
So my interpretation is water is more favorable than peroxide
"You can't do that" - challenge accepted
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That depends on kinetics, not thermodynamics. It's a completely different field.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
"Kinetics"
Been searching the text for mention of reaction speeds.
Big bold chapter title but i don't know what it was called.
Thank you
"You can't do that" - challenge accepted
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
WoW big concepts, very small head.
My end goal is to enter the reagents and products, temp, and pressure. And get delta g for a reaction before I start experimenting with my pressure
chamber.
My book contains a few formulas regarding pressure.
I'm having trouble understanding if the first one is simply used to demonstrate a concept, or if the second is required if the values are known.
1) ΔG = G⁰ + RTln(P)
2A) ΔG = G⁰ + RTln(Q)
2B) reaction: 1N2(g)+3H2(g)→2NH3(g)
Q = (PNH3)2 ÷ ( (PN2)1)
(PH2)2 )
Wikipedia adds to my misunderstanding because it doesn't list either of these formulas but instead shows
3) ΔG = U + pV - TS
This leads me to another question.
Can I use a compound's standard G⁰ to solve for U in equation 3 at standard pressure, then solve for ΔG at the pressure in question?
To increase my difficulty the reaction I want to experiment with uses solids, liquids, and gases.
"You can't do that" - challenge accepted
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Eq'n 1 is only applicable to a single gas.
Eq'n 2 is applicable for a regular reaction (but there should be a delta in front of both Gs. ΔG = ΔG⁰ + RTln(Q))
This allows you to calculate ΔG for a reaction at non-standard pressures and concentrations. ΔG⁰rxn can be found from free energies of formation.
To calculate ΔG at non-standard temperatures, you need ΔG = ΔH - TΔS (but note that enthalpy and entropy are not completely independent of
temperature).
I don't see how eq'n 3 would be useful.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
It appears you have fallen foul of DraconicAcid, he loves to correct the difference between kinetics and thermodynamics, he has done it to me twice!

That was a few years ago but the difference between the two is still fuzzy to me sadly.
"WoW big concepts, very small head." - I can relate
[Edited on 15-10-2022 by SplendidAcylation]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Reply deleted
[Edited on 16-10-2022 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Exactly what I need. No reason to worry, or complain
We're talking math. It's exact.
Almost only counts in horseshoes and hand grenades.
All the videos and lectures on this make it seem so simple. And the concept is, but the application is much more complex. Reminds me of Ms. Triplet's
ap calculus final
"1 question, 22 parts. Show your work. You have 3 hours"
"You can't do that" - challenge accepted
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by SplendidAcylation  |
It appears you have fallen foul of DraconicAcid, he loves to correct the difference between kinetics and thermodynamics, he has done it to me twice!

That was a few years ago but the difference between the two is still fuzzy to me sadly.
"WoW big concepts, very small head." - I can relate
|
Well, they are completely different. Try to think of it this way: thermodynamics can answer the question
“will I get X product?” But it won’t tell you whether getting to that product will take a second or (literally) a trillion years. That’s where
kinetics comes in.
This is where it’s helpful to look at a free energy diagram (shamelessly stolen from some website):
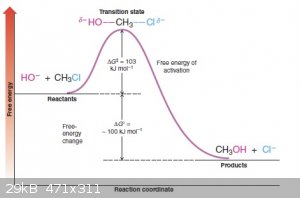
So looking at this diagram, you can see that the ΔG0 is the difference in energy between the reactants and products. In this case, it’s
negative, so the reaction is spontaneous. Great. However, there’s also the ΔG‡, otherwise known as Ea, or activation energy. This is
what tells you how fast the reaction is (kinetics!). A higher Ea means a slower reaction. A lower Ea indicates a fast reaction. Generally we supply
this energy by simply heating the reaction mixture, which is why (you may have been wondering) many “spontaneous” reactions still need to be
heated. Sometimes the Ea is too high to be overcome just by applying heat, though. It may be that your starting materials or products undergo
decomposition or side reactions at the temperatures required. In these cases, the reaction won’t work from a practical standpoint even if it is
thermodynamically favorable, but it may be possible to lower the activation energy by adding a catalyst. That’s what catalysts do: they provide an
alternative reaction pathway with a lower activation energy, thereby making the reaction proceed faster.
I like free energy diagrams because they combine thermodynamics and kinetics into one nice visual.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by Texium  | Quote: Originally posted by SplendidAcylation  |
It appears you have fallen foul of DraconicAcid, he loves to correct the difference between kinetics and thermodynamics, he has done it to me twice!

That was a few years ago but the difference between the two is still fuzzy to me sadly.
"WoW big concepts, very small head." - I can relate
|
Well, they are completely different. Try to think of it this way: thermodynamics can answer the question
“will I get X product?” But it won’t tell you whether getting to that product will take a second or (literally) a trillion years. That’s where
kinetics comes in.
This is where it’s helpful to look at a free energy diagram (shamelessly stolen from some website):
So looking at this diagram, you can see that the ΔG0 is the difference in energy between the reactants and products. In this case, it’s
negative, so the reaction is spontaneous. Great. However, there’s also the ΔG‡, otherwise known as Ea, or activation energy. This is
what tells you how fast the reaction is (kinetics!). A higher Ea means a slower reaction. A lower Ea indicates a fast reaction. Generally we supply
this energy by simply heating the reaction mixture, which is why (you may have been wondering) many “spontaneous” reactions still need to be
heated. Sometimes the Ea is too high to be overcome just by applying heat, though. It may be that your starting materials or products undergo
decomposition or side reactions at the temperatures required. In these cases, the reaction won’t work from a practical standpoint even if it is
thermodynamically favorable, but it may be possible to lower the activation energy by adding a catalyst. That’s what catalysts do: they provide an
alternative reaction pathway with a lower activation energy, thereby making the reaction proceed faster.
I like free energy diagrams because they combine thermodynamics and kinetics into one nice visual. |
Sorry about the slow response.
Nice explanation! Thank-you.
I like those diagrams too.
I tended to avoid any talk of kinetics because it seemed a bit mathematical and abstract to me, but now I see how important it is I guess I'd better
try harder 
I shall read the kinetics pages on chemguide, that should help.
|
|
|