Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Making Iron from Sand
https://www.youtube.com/watch?v=OPIUMpiV0IY
Pretty cool, not too often do we see trending videos in the YouTube section on chemistry
Saw one a while ago about the production of bronze, iirc.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
How hot does a “primitive” furnace like this get?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Yeah. This guy is good.
A lot of skill in mandling clay and other bushcraft techniques.
Fundamental to a lot of what he does is having good procedures for making charcoal.
I watched another one of his recently where the source material was bacterial sludge that had concentrated iron (I think as the sulfide). It was a
pretty good effort with a nice result. He made an iron knife.
To answer your question, just look up the melting point of iron.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
And then look up the melting point of the iron/ carbon eutectic.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Is this for making steel?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Cast Iron has a carbon content of typically 4.3% by mass. That is the eutectic point of the carbon iron system. Eutectic temperature is 1147C
Properties of cast iron differ significantly from steel.
Steels of various kinds have properties determined in part by the microstructure which is related to the heating/cooling history around the eutectoid
point (0.76%C and 723°C)
At this concentration of carbon the steel melts at around 1500°C. Cooling passes through the austenite phase and then the ferrite phase.
A higher melt temperature is required in practice. Typical slag temperature is around 1630°C, and it needs to be molten to effectively carry away
impurities.
I have not watched the video you posted, but the previous one I did watch is presumably similar. I would estimate that he achieved a temperature just
shy of 1600°C which is just enough to have an effective slag. And my guess is that he ended up with a high carbon steel rather than a cast iron:
maybe 1% carbon.
Of course this is all guessing, but it does give a ball-park answer to your question.
The iron-carbon phase diagram is a complex beast. (Simple version below) But it has features that mean that steel properties are very manipulable.
This is one of the reasons why steel is so ubiquitous.
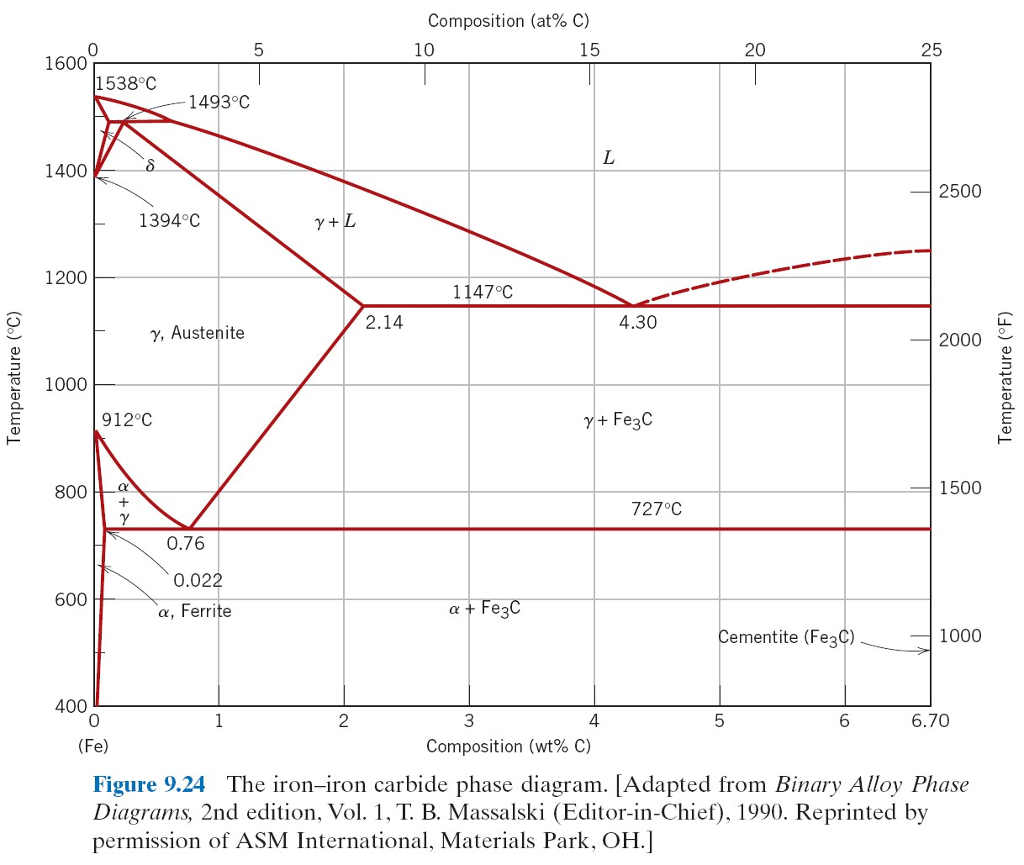
[edit - post better diagram]
[Edited on 4-9-2022 by j_sum1]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It's complicated but what you get out of a primitive furnace is "pig iron" or "cast iron" which has a lot of carbon in it.
To turn it into steel you need to remove the carbon some how. It's "unhelpful" that the stuff we call "cast iron" actually has less iron in than steel
has.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Quote: Originally posted by j_sum1  | Cast Iron has a carbon content of typically 4.3% by mass. That is the eutectic point of the carbon iron system. Eutectic temperature is 1147C
Properties of cast iron differ significantly from steel.
Steels of various kinds have properties determined in part by the microstructure which is related to the heating/cooling history around the eutectoid
point (0.76%C and 723°C)
At this concentration of carbon the steel melts at around 1500°C. Cooling passes through the austenite phase and then the ferrite phase.
A higher melt temperature is required in practice. Typical slag temperature is around 1630°C, and it needs to be molten to effectively carry away
impurities.
I have not watched the video you posted, but the previous one I did watch is presumably similar. I would estimate that he achieved a temperature just
shy of 1600°C which is just enough to have an effective slag. And my guess is that he ended up with a high carbon steel rather than a cast iron:
maybe 1% carbon.
Of course this is all guessing, but it does give a ball-park answer to your question.
The iron-carbon phase diagram is a complex beast. (Simple version below) But it has features that mean that steel properties are very manipulable.
This is one of the reasons why steel is so ubiquitous.

[edit - post better diagram]
[Edited on 4-9-2022 by j_sum1] |
What’s in the 2.4’— area?
|
|
|