Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Measuring distillation column performance
Question: what data should be collected to calculate the theoretical plates of my distillation apparatus? How should this data be analyzed to
calculate the maximum my apparatus is capable of?
I plan on setting up a series of ethanol distillations to collect the needed data.
Each series will be 3 concentrations by weight with 3 different columns.
I'm going to try using different packing materials and compare that to my current unpacked column.
By a great chance of luck, I have acquired some
1mm borosilicate glass beads.
Situation:
Over the last 2 years, I have performed a lot of distillation. So much I've built an esp-based controller to help manage and collect data regarding
the procedures I perform.
I am still perfecting the software part but in short, 5 temp probes, 4 PID controllers, and 3 relays all rolled up into 1 package.
Using scripts to work the controllers and an overly complicated setup
I can get very good separation with little monitoring and a short column.
Temp 1 is the hotplate temp
Temp 2 is the solution temp
Temp 3 is the lower 3rd of the 100mm fractional column.
Temp 4 is the upper 3rd of the column
Temp 5 is the head temp.
Relay 1 hotplate, 20-second cycle time.
Relay 2 lower column fan.
Relay 3 upper column fan.
Pid 1 controls relay 1 directly. It has a max temp limit for the hotplate( to protect my glass) and works the solution temperature. But can be
adjusted up or down by the other PID's
Pid2 controls the lower column fan directly and influences pid1 when the fan is no longer capable of cooling the column to the desired temp.
Pid3 controls the upper column fan directly and influences pid 2.
Pid 4 monitors the head temp and has first control over PID1(hotplate). And influences PID3 to a lower temp
Order of operations:
User sets operational profile. ( enters fraction temperatures.) System starts
1) PID4 engages and influences PID1 to increase Temp1 until the distilation head reaches the first fraction temp, when Temp5 is equal to or overshoots
the desired fraction temp, PID3 is influenced to a lower temp.
2a) PID3 initially keeps the top of the column cooled to a temp greater than Temp5, until influenced, when Temp4 overshoots, PID2 is influenced to a
lower temp.
2b) PID2 initially keeps the bottom of the column cooled to a temp greater than Temp5 until influenced. When Temp3 overshoot, PID1 is influenced to a
lower temp
3) When Temp5 can not be maintained at the fraction temp without forcing PID3 and PID2 below the fraction temperature, activate "end of fraction"
alarm.
4)next fraction profile is loaded and run. GOTO step 1
The temperature of each section of the apparatus is recorded so I can see what adjustments are needed and what controls the user needs to write a
script. It also allows me to see the temp gradients on my apparatus. Which are nothing like the textbooks show. Usually less than a 10th of a degree.
The greater my separation, the lower my distillation rate. Detecting the end of the fraction has by far been the hardest part. But I have
successfully generated 95% ethanol multiple times from different concentrations. I have also generated 68% nitric acid but dam it's slow.
"You can't do that" - challenge accepted
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I find this subject very interesting.
However I have no idea what a column fan is or what it does.
I'll be watching this thread so I can hopefully learn a few things about distillation.
I'd love to figure out how many plates my big still has.
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I did not calculate / determine that, but it is done by distilling a mixture of 2 solvents and determine the concentration of both in condensate:
http://www.phywe-es.com/index.php/fuseaction/download/lrn_fi...
Attachment: P3031501.pdf (531kB)
This file has been downloaded 283 times
From my experience, 1 m long Hempel column with internal 30 mm diameter packed with glass Raschig rings and variable ratio reflux distillation head
with ratio 10:1 produced very pure ethanol with only traces of methanol and ethylacetate. Starting material was crushed apples + water + cane sugar,
first 2 simple distillations from bigger still (after 1st distillation 40 vol%, after 2nd 80 vol%) and final column distillation from 80 vol% ethanol
to azeotrope. My friend Bedlasky performed gas chromatography, here for more:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Quote: Originally posted by SWIM  | I find this subject very interesting.
However I have no idea what a column fan is or what it does.
|
It is a fan that blows over the column to cool it and increase reflux. I placed a cardboard box around the top and bottom half of my column so I can
cool the top separately from the bottom as well as provide the insulation needed for the vapor to reach the head. The extra cooling of the column is
needed when it becomes flooded. By increasing the reflux I can maintain proper seperation at the expense of flow rate.
I have been able to increase my distillation rate by having more heating watts and cooling watts. Keeping my column just shy of flooding maximizes
flow rates but is time-consuming to watch and adjust, hence the controller.
By collecting 5 data points, and knowing the wattage of my hotplate I can calculate the amount of energy entering the system.
By monitoring the solution temperature I can estimate how much energy is leaving the system. This is the key to detecting fractions. As one fraction
finishes, the amount of energy in the solution begins to increase as the loses decrease.
It was cheaper than buying a longer column, the thermocouples, relays and custom pcb was less than 100 bucks. And a lot more fun.
| Quote: |
I did not calculate / determine that, but it is done by distilling a mixture of 2 solvents and determine the concentration of both in condensate:
|
Thank you. That is exactly what I have been searching for.
That is an absolutely beautiful setup, wish my column was that big.
"You can't do that" - challenge accepted
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Fery  | I did not calculate / determine that, but it is done by distilling a mixture of 2 solvents and determine the concentration of both in condensate:
http://www.phywe-es.com/index.php/fuseaction/download/lrn_fi...
From my experience, 1 m long Hempel column with internal 30 mm diameter packed with glass Raschig rings and variable ratio reflux distillation head
with ratio 10:1 produced very pure ethanol with only traces of methanol and ethylacetate. Starting material was crushed apples + water + cane sugar,
first 2 simple distillations from bigger still (after 1st distillation 40 vol%, after 2nd 80 vol%) and final column distillation from 80 vol% ethanol
to azeotrope. My friend Bedlasky performed gas chromatography, here for more:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15... |
This is also just what I was looking for.
Thanks.
A meter of Raschig rings is some pretty serious gear. I'm not surprised you're getting such impressive results with that and a 10:1 reflux ratio.
Is that a jacketed column?
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi SWIM, the column is not jacketed. Its price was something like 20 EUR, jacketed are much more expensive (more than 5 times). I insulated it by
myself, although certainly not so good as vacuum jacket.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Still sounds like great prices for glassware, but I see you're in a Czechoslovakia.
You guys are famous for your glass.
Even out here in California.
|
|
|
wg48temp9
National Hazard
   
Posts: 783
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Rainwater:
Have you used your 1mm glass beads as comum packing yet?
I would assume they would flood easily as the gaps between 1mm beads would be blocked by liquid held there by surface tension.
By cooling the column your using it as both a column and a condenser. To maximise the number of theoretical plates insulate the column and add a
reflux condenser to the top with a device between the two that diverts a small fraction of the reflux (about 10%) to your output.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  | Still sounds like great prices for glassware, but I see you're in a Czechoslovakia.
You guys are famous for your glass.
Even out here in California. |
Bohemian glass was famous before California existed. :-)
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I agree about Bohemian glass, but face it, almost everything was famous before California existed.
Prince Albert was.
But probably not that piercing he invented.
[Edited on 20-5-2022 by SWIM]
But congratulations on knowing that they had Bohemian glass before the mid 19th century.
Go grab yourself a Frobisher and Gleason raspberry ice lolly and celebrate. [-]_[-]
[Edited on 21-5-2022 by SWIM]
[Edited on 21-5-2022 by SWIM]
[Edited on 21-5-2022 by SWIM]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Not yet. This will be my first time using a packed column.
I planned on 3 test. 1 empty column (control) measuring collection rate.
2 layered packing. Cotton ball, beads, space - repeat
3 column filled with beads to the top.
That's the general idea.
The primary focus of my design is the maintenance of the temperature gradient of my column
By over heating the boiling flask, the pids start to lower the heating in incurments to maintain the ideal gradient across the column. As the top
cooler reaches its limit, the lower cooler kicks in. When it reaches its limit, the hotplates power is lowered until the system reaches balance.
As the boiling point of the solution increases, the controller adapts to maintain the gradient.
Which gives me a faster flow rate while maintaining separation.
Ramble:
When I was using just a hotplate, I had to constantly monitor the apparatus to ensure the head was at the target temperature to ensure good
separation.
From that, I progressed to using a standard pid to control the hotplate based on head temperature, which produced great separation at very slow rates
and would cause oscillation, which would bring condensation to a stop.the temperature at the head would reach the desired value but no condensation
would come over. The controller i have does not have a "feed" setting to pump a little more energy into the system continusily. And it managed to
crack a 1L rbf.
On a cold day outside, I discovered that by cranking up the heater wattage, I could achieve the same separation at a faster rate, which led me to
my latest experiments
I already had a spair diy kiln controller, and with a few modifications to the hardware and a complete rewrite of the software, I started using it to
operate my distilations.
Soon after, I reached the limit of my short column. Flooding is a minor issue, but by balancing the heating and cooling, I better control the
temperature gradient of my column, getting as close to ideal separation as I am able to measure. When first started, it will flood the column, as the
controlers start tuning in, this stops.
The apparatus performed at various rates through a run, but separation is great.
"You can't do that" - challenge accepted
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I cool from the top like WG48.
The thing about cooling the column is (partly) that it means there will be more condensate running down the lower half than the upper half and that
means the top part of the column will have less reflux.
You cool just above the column and the whole column can be run at maximum reflux.
These partial takeoff heads cool the distillate and return to the column from the top. They can be set for total reflux while you get the power output
right and then you open the collection valve to set the reflux ratio. They do a great job for almost any distillation except the really high-powered
hard to do stuff.
They're also not too expensive used if you shop around for a while or go through Dr Bob, who usually has one or two up his sleeve.

These Electromagnetic heads are for really high reflux ratios, and give a constant ratio regardless of vapor throughput rate.
They'll do ridiculous things like 60:1 reflux ratios, but there aren't too many things you'd need one of these for.
Maybe separating xylene isomers, or separating the constituents of (edit: add complex, difficult to separate) essential oils.
If you don't have dozens of theoretical plates you probably don't need one.
At least this is what I hear. Haven't had much experience with high-powered distillations yet.
Oh yeah, these need an electromagnet and timer to work.

[Edited on 21-5-2022 by SWIM]
[Edited on 21-5-2022 by SWIM]
[Edited on 21-5-2022 by SWIM]
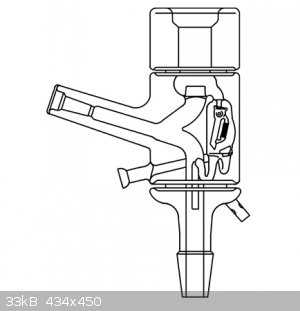
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Very nice.
So the bottom valve is the reflux control
The top is a vacuum port
Not sure about the middle one. Looks like another gas port with a 3way directional valve.
Hopefully soon, I will be able to set up everything and post some data. Would like to compare notes and see if this idea can compete with professional
apparatus.
"You can't do that" - challenge accepted
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
middle valve is used to detach receiving flask during distillation and replace the flask with new one (without interrupting the distillation either
the vacuum in the distillation part)
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
My 2c ;
+1 that 1mm MAY be too small due to surface tension etc.
so it will be interesting to see what happens.
+1 that the column should perform best with insulation and a reflux condenser.
with a 20s cycle time for heating, I suggest a water or oil bath to reduce variations in vapour speed
I am dubious of closed-loop control of heating power based on stilhead temperature,
heating bath temperature regulation may be simplest.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Fery  | | middle valve is used to detach receiving flask during distillation and replace the flask with new one (without interrupting the distillation either
the vacuum in the distillation part) |
Yes, that's it.
They do make ones without the extra valves for vacuum work, but the price is similar used so it's probably best to get one with the extra valves. They
are much more common and really save trouble taking fractions under vacuum.
I'm not trying to discourage your experiments here. Just thought it would be a good place to show what sort equipment is most common for these uses.
I do know glassware, but there are engineers and chemists on here that understand the applications far better than I ever will.
And there are a lot of more esoteric stills out there. lots of interesting custom jobs actually made by our members.
Especially the ones who like a bit of moonshine.
EDIT: and yes, I'd run a few distillations under conditions like your tests if you like.
I've been planning to drag out some of my fancier gear for some tests and doing a few runs with the kind of stillhead shown in the first picture would
be good as a refresher course for me.
I've got one in 14/20 somewhere that I suspect might be similar in capacity to the glassware you're using.
I've got a 24/40 too. It has broken vacuum fraction cutting tubing but I replaced it with hosing and it works fine.
I bought like 3 24/40 PTO heads a few years back, and every one was broken in the mail (buying from amateurs on Ebay is always a bit of a crapshoot on
shipping. Bigger sellers on Ebay are much safer.
So's Dr Bob on here, who is no amateur.)
I finally gave up and just kept the last one as it was only that side vertical vacuum tube that was broken and I just stick tubing on it as a bridge
when I need to.
[Edited on 21-5-2022 by SWIM]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
After 10 different distillation runs using the column 3 set as described before i have concluded that the improvements I made to my temperature
controller, and not the extra cooling are they only factor which has improved my separation.
With a temperature resolution of 0.001c and monitoring the temperature gradient across the column.
The improvements i believed i was getting were errors in my measurements.
By removing the extra cooling and insulating the columns my apparatus was able to lock-in more quickly to the desired temperature and detect the
fractions more effectively.
Distillation rates were not affected.
"You can't do that" - challenge accepted
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Here is a good read on the original question
https://www.thermopedia.com/content/703/
"You can't do that" - challenge accepted
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@RW:
You familiar with the concept of Number of Theoretical Plates?
Very nice study, BTW! Mi gusto mucho...
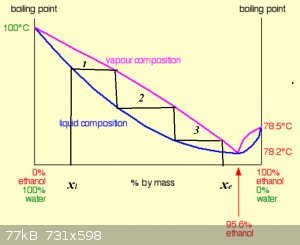
Distillation from xi (pot) to xe (distillate) in 3 theoretical plates (TP).
Obviously the higher the number of TP, the higher the separation power of a column/system.
[Edited on 11-12-2022 by blogfast25]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I'm still studying, most of the material is repetitive and each time I review it I understand a little more. So the logical thing is to keep reviewing
and experimenting.
I've learned that theoretical plates are not set in stone for any apparatus, both plate count and efficiency form the basics of separation power. That
plugged into a complex equation i don't understand yet equals the magic of seperation.
I currently have multiple fillers out for a reflux control apparatus. Them things(glass) are way overpriced.
I can use a 1 L RBF 200mm vigreux and distill 5% ethanol to 95% using 85 watts.
Very very omg that's slow. Like 100ml in 8 hours.
By increasing the heat input by 10% product purity drops to 75%
10% more and purity drops to 40%
Now by changing to an insulated 400mm packed column with a vigreux column on top,
I can increase heating to 155watts and get purity of 95%
Increasing heat by 5% purity drops to 93% 5%more and purity drops to 90%
The challenge is trying to express mathematical what is going on
"You can't do that" - challenge accepted
|
|
|
VeritasC&E
Hazard to Others
  
Posts: 176
Registered: 29-1-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rainwater  | I'm still studying, most of the material is repetitive and each time I review it I understand a little more. So the logical thing is to keep reviewing
and experimenting.
I've learned that theoretical plates are not set in stone for any apparatus, both plate count and efficiency form the basics of separation power. That
plugged into a complex equation i don't understand yet equals the magic of seperation.
I currently have multiple fillers out for a reflux control apparatus. Them things(glass) are way overpriced.
I can use a 1 L RBF 200mm vigreux and distill 5% ethanol to 95% using 85 watts.
Very very omg that's slow. Like 100ml in 8 hours.
By increasing the heat input by 10% product purity drops to 75%
10% more and purity drops to 40%
Now by changing to an insulated 400mm packed column with a vigreux column on top,
I can increase heating to 155watts and get purity of 95%
Increasing heat by 5% purity drops to 93% 5%more and purity drops to 90%
The challenge is trying to express mathematical what is going on |
Hi Rainwater!
Is there a specific reason/utility/purpose to adding a vigreux above a packed column or is it just that you don't have more column length and are
using it to increase the fractionation length?
Would it make any difference to put the vigreux beneath the packed column for instance?
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
More length.
I did not try placing the vigreux columns on the bottom. From my current understanding
I do not believe that would add any benefit.
I fell for the unspoken youtube myth
A non-insulated looks good on camera,
but cripples your separation.
An insulated column decreases the required heating and
improves separation
The absolute best literature I found about the physical process of fractional distillation is
...digging...
http://www.sciencemadness.org/talk/viewthread.php?tid=12409
When I started this series of experiments, my understanding of the true process was seriously flawed.
I learned that the true process of distillation has nothing to do with a condenser.
And that if your condenser is too cold, it can impair the process
To sum up what I read in the article.
The primary transfer of heat is by the vaporization and condensation of the mixture.
The higher boiling component, having more heat of vaporization, when condensing
transfers it's latent energy of heat into the less boiling component.
One important note from my experiments was that when the controller was properly locked in, the fans would turn off completely,
Balancing the heating with the takeoff has provided the best results.
I got a new peace of glassware last week.
Have not had time to use it yet but im really looking forward to it.
Its a cheap(ish) reflux head.
A 24/40 claisen adapter with a takeoff stopcock.
"You can't do that" - challenge accepted
|
|
|
VeritasC&E
Hazard to Others
  
Posts: 176
Registered: 29-1-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rainwater  | More length.
I did not try placing the vigreux columns on the bottom. From my current understanding
I do not believe that would add any benefit.
I fell for the unspoken youtube myth
A non-insulated looks good on camera,
but cripples your separation.
An insulated column decreases the required heating and
improves separation
The absolute best literature I found about the physical process of fractional distillation is
...digging...
http://www.sciencemadness.org/talk/viewthread.php?tid=12409
When I started this series of experiments, my understanding of the true process was seriously flawed.
I learned that the true process of distillation has nothing to do with a condenser.
And that if your condenser is too cold, it can impair the process
To sum up what I read in the article.
The primary transfer of heat is by the vaporization and condensation of the mixture.
The higher boiling component, having more heat of vaporization, when condensing
transfers it's latent energy of heat into the less boiling component.
One important note from my experiments was that when the controller was properly locked in, the fans would turn off completely,
Balancing the heating with the takeoff has provided the best results.
I got a new peace of glassware last week.
Have not had time to use it yet but im really looking forward to it.
Its a cheap(ish) reflux head.
A 24/40 claisen adapter with a takeoff stopcock.
|
Thanks a lot for sharing these insights!
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
From my own experiments I suggest that it is very difficult to accurately determine the number of theoretical plates of a column using water:ethanol
once you have a still capable of achieving anything near azeotropic concentration.
eg the difference between 10 and 11 plates gives only a small difference in concentration,
I was unable to get repeatable results due to this,
and due to lack of full control over all variables, especially reflux ratio.
A simpler approach is to build the best still that you can and experiment with different column packings and reflux ratios.
The best that I've managed so far is :
using different sized glass spheres I verified to my own satisfaction that performance IS proportional to
the surface area of the packing (plus the column wall) .
I've not done enough work to be able to calculate the effect of different reflux ratios, for practical purposes
(time, plus heating cost to produce product)
about 3:1 seems reasonable.
I'm sure others with more experience can correct me 
PS a Vigreaux column seems to me to offer poor fractionating efficiency, but if packed it is better than a plain column as the indentations
significantly reduce channeling down the wall.
[Edited on 2-2-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|