SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
UV Photolysis
Any of you guys done UV photolysis tests on simple organic chemicals before? Namely acetate or ammonium compounds, starches, cellulose, triglycerides,
soap, whatever. I might try shining a UV light on those for a while to see if anything fun happens and I wanna know if anyone's tried that before (or
knows of any other cool photolytic reactions).
I have also read that some transition metal oxides, such as zinc and titanium, have some sort of photocatalytic effect.
|
|
|
sauveurdumonde
Harmless

Posts: 33
Registered: 13-7-2021
Location: Canada
Member Is Offline
|
|
Nothing major, but I've tried UV photolysis of silver (i) chloride to silver, so it would dissolve in acetic acid. It worked- okay. The UV light I was
using was mostly violet and UV-A though, so a bit weak 
"There is a shadow of a nation behind you, the hope of a people... yet it may not matter. The Divide still stands against us."
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by sauveurdumonde  | Nothing major, but I've tried UV photolysis of silver (i) chloride to silver, so it would dissolve in acetic acid. It worked- okay. The UV light I was
using was mostly violet and UV-A though, so a bit weak  |
ah yeah, I've heard a lot of the silver salts are very sensitive to light
[Edited on 2/10/2022 by SnailsAttack]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The compounds you listed ( acetate or ammonium compounds, starches, cellulose, triglycerides, ) are not going to work well.
They don't absorb UV except at rather short wavelengths and I doubt you are using those.
The "soap" is a bit poorly defined. It may have dyes and perfumes in which are strong UV absorbers.
|
|
|
sauveurdumonde
Harmless

Posts: 33
Registered: 13-7-2021
Location: Canada
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  |
Quote: Originally posted by sauveurdumonde  | Nothing major, but I've tried UV photolysis of silver (i) chloride to silver, so it would dissolve in acetic acid. It worked- okay. The UV light I was
using was mostly violet and UV-A though, so a bit weak  |
ah yeah, I've heard a lot of the silver salts are very sensitive to light
[Edited on 2/10/2022 by SnailsAttack] |
Yes, the freshly precipitated AgCl was a very bright white but after a few minutes it turned a dark, silvery black
"There is a shadow of a nation behind you, the hope of a people... yet it may not matter. The Divide still stands against us."
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
You can get lots of different wavelength UV sources cheap these days, but if you mess around with UVC get some proper UVC glasses.
Those short wavelengths aren't stopped effectively by ordinary polarized sunglasses.
Keep your skin covered too. Those UVC bulbs will give you a sunburn that smells like barbecued pork, and that can't be good.
That's for UVC around 250 Nm. The bulbs that have a second peak at 180 or so also make ozone from the air which is a whole other problem to deal with.
Also, UVC doesn't go through Pyrex very well, much worse transmission than UVA/UVB.
Either you have to use lots of wattage or use quartz containers (expensive unless you get lucky on Ebay)
I guess what I'm saying is stick to the UVA/UVB wavelengths unless you really have a good reason to mess with the shorter ones.
But curiosity can be a good reason if you're curious enough, just be aware that that stuff is pretty harsh.
You can make your own chlorinated hydrocarbons, but some of the byproducts are pretty nasty so there doesn't seem to be much interest in doing this at
home.
There are a few write-ups on here for making carbon tetrachloride with UVC acting on chloroform and chlorine gas, but they were using UVC from a 400
watt bulb and it looked like quite a production.
Not having a proper photochemical reactor complicates things a bit.
Magpie got good results though.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by SWIM  |
Keep your skin covered too. Those UVC bulbs will give you a sunburn that smells like barbecued pork, and that can't be good.
That's for UVC around 250 Nm. |
That smell when you expose fingers even to a few seconds of an unprotected low power UVC lamp is surprisingly strong. To my nose its similar to the
smell of burnt skin from briefly accidently touch something that very hot.
Does anyone know what compound/s causes it ?
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Bonee
Harmless

Posts: 29
Registered: 20-12-2010
Member Is Offline
Mood: No Mood
|
|
I would put my bet on similar to heat denaturation of keratin proteins and subsequent cleaving of disulfide bonds in it.
Of course this is a gross simplification, but see ref:
tyrosine mediated uv absorbtion and disulfide bond breakage.
https://pubmed.ncbi.nlm.nih.gov/26997094/
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
UVC
I bought a 60-watt UVC LED "corn-cob" styled bulb in the early days of COVID.
This to kill any COVID on surfaces. Whether or not this was effective is unknown
to me because less than a minute after turning the bulb on something smelled like
it was burning. Fortunately I wasn't in the room and the bulb came with a fixture
with a remote control to allow me to operate without exposure. The reflection
from the room was intensely violet-white light. Nothing like I'd ever seen with a
black lamp. I haven't used this thing since.

[Edited on 2022/2/12 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
People seem to be ignoring the important factor here.
Here's the bit from the OP where the chemicals are specified.
"UV photolysis tests on simple organic chemicals before? Namely acetate or ammonium compounds, starches, cellulose, triglycerides,"
Let's pick ethyl acetate as an example, because the data is easy to find.
Here's the UV spectrum (from here)
https://us.vwr.com/store/product/4542490/ethyl-acetate-99-9-...
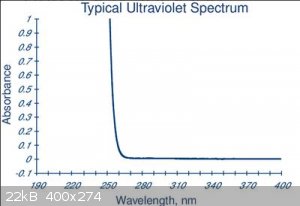
There's no absorption of light above 270 nM
So there's no possible effect of UV / visible light above 270nM because the light just goes through.
And there are not many convenient UV sources in the region where the stuff absorbs- the obvious option is a low pressure Hg lamp emitting lots of 254
nM UV. (The 181 nM emission is blocked by the oxygen in the air so it's a red herring for photolysis)
And here's the transmission spectrum of Pyrex glass.
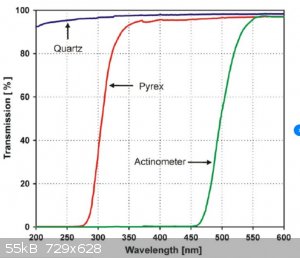
Showing that there is no transmission below about 280nM
So no light that is long enough wavelength to go through glass is short enough to be absorbed by an acetate.
You can do the same thing with ammonium compounds , starches etc.
Unless you are working with compounds with a good chromophore, you can't do photochemistry in glass containers.
[Edited on 12-2-22 by unionised]
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Apparently that may be a good bet LOL. The wiki entry on keratin suggests that the pungent smell of burnt hair and skin is due to volatile sulphur
compounds from the decomposition of the disulphide bonds. Though I would not describe the smell as pungent but to me it smells very distinct.
The chew sticks my dog likes are made from cow or pig skin, I probably have some of my hair clippings in the bathroom and I could make some nail
clippings to see if they all give off a similar smell when exposed to UVC or touched with a hot soldering iron.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Wow, so Lem1 and Magpie were wasting their time using an external 400 watt mercury vapor source to chlorinate chloroform to carbon tetrachloride in
glass RBFs?
Having both quartz wells for my photo-chemical reactor and long thin uvc sources that are shaped like test tubes and can be spigotted into the necks
of glass RBFs I've never tried to do it that way, but I assumed they knew what they were doing.
So How'd they get CCl4 then?
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by MadHatter  | I bought a 60-watt UVC LED "corn-cob" styled bulb in the early days of COVID.
This to kill any COVID on surfaces. Whether or not this was effective is unknown
to me because less than a minute after turning the bulb on something smelled like
it was burning. Fortunately I wasn't in the room and the bulb came with a fixture
with a remote control to allow me to operate without exposure. The reflection
from the room was intensely violet-white light. Nothing like I'd ever seen with a
black lamp. I haven't used this thing since.
|
That LED corn cob looks like it has about 360 LEDs, if they are UVC LEDs I would expect it cost more than £100. UVC LEDs are expensive compared to
longer wavelength LEDs.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Texium
|
Thread Moved
12-2-2022 at 08:57 |
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  | Wow, so Lem1 and Magpie were wasting their time using an external 400 watt mercury vapor source to chlorinate chloroform to carbon tetrachloride in
glass RBFs?
Having both quartz wells for my photo-chemical reactor and long thin uvc sources that are shaped like test tubes and can be spigotted into the necks
of glass RBFs I've never tried to do it that way, but I assumed they knew what they were doing.
So How'd they get CCl4 then? |
The chlorination of chloroform is energetically favoured and is a "classic" free radical reaction.
In the absence of inhibitors you can have quantum yields of 1000 or whatever.
More importantly, Chlorine is yellow.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by SWIM  | | Keep your skin covered too. Those UVC bulbs will give you a sunburn that smells like barbecued pork, and that can't be good. |
um, awesome
Quote: Originally posted by SWIM  | You can make your own chlorinated hydrocarbons, but some of the byproducts are pretty nasty so there doesn't seem to be much interest in doing this at
home.
There are a few write-ups on here for making carbon tetrachloride with UVC acting on chloroform and chlorine gas, but they were using UVC from a 400
watt bulb and it looked like quite a production. |
400 watts? good god. i have heard about chlorinating hydrocarbons this way, that could be cool. i'd like makin my own DCM.
Quote: Originally posted by unionised  | There's no absorption of light above 270 nM
So there's no possible effect of UV / visible light above 270nM because the light just goes through.
And there are not many convenient UV sources in the region where the stuff absorbs- the obvious option is a low pressure Hg lamp emitting lots of 254
nM UV.
So no light that is long enough wavelength to go through glass is short enough to be absorbed by an acetate.
You can do the same thing with ammonium compounds , starches etc. |
ah, that's too bad.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by SnailsAttack  | Quote: Originally posted by SWIM  | You can make your own chlorinated hydrocarbons, but some of the byproducts are pretty nasty so there doesn't seem to be much interest in doing this at
home.
There are a few write-ups on here for making carbon tetrachloride with UVC acting on chloroform and chlorine gas, but they were using UVC from a 400
watt bulb and it looked like quite a production. |
400 watts? good god. i have heard about chlorinating hydrocarbons this way, that could be cool. i'd like makin my own DCM. |
Easier said than done. Free radical chlorination of methane will give you a mixture of chloromethane, DCM, chloroform, and carbon
tetrachloride. Also the mixture of methane and chlorine is explosive. Also chloromethane is a highly toxic gas. Really it’s a reaction that’s best
left to industrial facilities with specialized reactors and the ability to separate all the products efficiently.
Conversion of chloroform (either purchased or made by the haloform process) to carbon tetrachloride, on the other hand, is a lot more practical and
useful, and has been done by a few members here. Look it up if you’re interested. Still definitely quite the undertaking, and carbon tet is pretty
nasty stuff.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
" Scheele wrote a description of chlorine... chemists suggested that the gas might be a pure element, and this was confirmed by Sir Humphry Davy .who
named it after the Ancient Greek χλωρός (khlōrós, "pale green") because of its colour."
|
|
|
khlor
Hazard to Self
 
Posts: 95
Registered: 4-1-2014
Location: Who knows, really...
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  | You can get lots of different wavelength UV sources cheap these days, but if you mess around with UVC get some proper UVC glasses.
Those short wavelengths aren't stopped effectively by ordinary polarized sunglasses.
Keep your skin covered too. Those UVC bulbs will give you a sunburn that smells like barbecued pork, and that can't be good.
That's for UVC around 250 Nm. The bulbs that have a second peak at 180 or so also make ozone from the air which is a whole other problem to deal with.
Also, UVC doesn't go through Pyrex very well, much worse transmission than UVA/UVB.
Either you have to use lots of wattage or use quartz containers (expensive unless you get lucky on Ebay)
I guess what I'm saying is stick to the UVA/UVB wavelengths unless you really have a good reason to mess with the shorter ones.
But curiosity can be a good reason if you're curious enough, just be aware that that stuff is pretty harsh.
You can make your own chlorinated hydrocarbons, but some of the byproducts are pretty nasty so there doesn't seem to be much interest in doing this at
home.
There are a few write-ups on here for making carbon tetrachloride with UVC acting on chloroform and chlorine gas, but they were using UVC from a 400
watt bulb and it looked like quite a production.
Not having a proper photochemical reactor complicates things a bit.
Magpie got good results though.
|
Heed his advice, once a novice on the world of photochemistry ,I had the pleasure of using a mercury vapor lamp, luckily I decided to put glasses on
before turning it on... I admired its beauty and did not notice the deadly rays and the smell of ozone... it was for a short time(no more than 5 min),
but by the end of that day I was suffering from that thing when you have to much sun(soorrry can't remember, getting old)... and worse, I got
"sunburns" so bad my skin took a week to heal, it is like I took a whole week's worth of sun... cover your skin as well as your eyes(WITH PROPER UVC
RATED GLASSES) when working with UVC, not even factor million sunblock helps.
"NOOOOOO!!! The mixture is all WROOOOOOONG!"
|
|
|
khlor
Hazard to Self
 
Posts: 95
Registered: 4-1-2014
Location: Who knows, really...
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SnailsAttack  | Any of you guys done UV photolysis tests on simple organic chemicals before? Namely acetate or ammonium compounds, starches, cellulose, triglycerides,
soap, whatever. I might try shining a UV light on those for a while to see if anything fun happens and I wanna know if anyone's tried that before (or
knows of any other cool photolytic reactions).
I have also read that some transition metal oxides, such as zinc and titanium, have some sort of photocatalytic effect.
|
Take a look atbTiO2 photocatalyst and other compounds like that, you may not be able to do photolysis, but, you can still do come pretty interesting
photochemistry , true, not everything you shine UV(A, B or C) will change, but that is what catalysts are for. I myself did some with chlorine(no
catalysts needed there) but I plan to play with plenty other things, copper compounds, ethanol, some oxides.... but whatever the case, I think that
Titanium dioxide would be a nice start, cheap, easily available, non toxic , non poisonous... I have a bag myself, tried some stuff in the past, not
greate results... try different temperatures as well, you may get different levels of photoactivity.
"NOOOOOO!!! The mixture is all WROOOOOOONG!"
|
|
|