EliasExperiments
Hazard to Self
 
Posts: 57
Registered: 3-3-2020
Member Is Offline
|
|
Experiments with liquid air
Hi I've made a video about experiments with liquid air:
https://youtu.be/thQ--TkEa6A
What experiment do you like most, do you have any ideas for better experiments?
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Because the cotton reacted so nicely i think it would be nice to see liquid air soaked into more stuff. Like thick fabric stuff (maybe carpet),
styrofoam and charcoal
|
|
|
EliasExperiments
Hazard to Self
 
Posts: 57
Registered: 3-3-2020
Member Is Offline
|
|
Yeah I was planning to do a video on liquid oxygen anyway and have your ideas noted. Thank you for the feedback! :-)
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Cool video.
Could be fun testing the impact sensitivity of some of those combos.
Also, try hitting frozen (silicone?) rubber with a hammer. Watch out though, it will throw off splinter like it was glass, while also having a wicked
rebound. At least that's what happened when I did it with a large blue rubber bung once. Not sure if natural rubber will act the same.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Turn on a CFL bulb and dip it into the liquid air.
Also, is it possible to condense the Hg in a CFL, and break it open and collect droplets of Hg?
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Not that I have any idea what would happen but what if you mixed liquid air with liquid propane? I'd hate for anything harmful to happen, especially
if the liquid air was oxygen rich. Maybe even a teaspoon would be too dangerous, even with flack and hearing protection. Or maybe being so cold the
reaction less fierce?
I'd be extra cautious experimenting with liquid oxygen, you don't want to be a life after deflagration or detonation storyteller.
Propane freezing point
-306.4°F
-188°C
Flash point of propane −104°C (−155°F)
Liquid oxygen boiling point
-297.3°F
-183°C
[Edited on 21-12-2020 by Morgan]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
you can get nearly 100% yield of liquid O2 if you replace the liquid N2 with atmospheric oxygen. all you need is some insulating
sleeves, copper pipes that about 1m long and (one is smaller than the other) a wire to wrap a spiral in smaller pipe to center it, so to make heat
exchanger and pump dry air into the dewar flask through it. think of a simple water cooled condenser used for distillation. all of them are sold at AC
( HVAC ) shops.
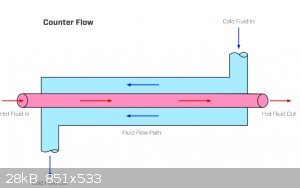
[Edited on 21-12-2020 by rockyit98]
"A mind is a terrible thing to lose"-Meisner
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by Morgan  | Not that I have any idea what would happen but what if you mixed liquid air with liquid propane? I'd hate for anything harmful to happen, especially
if the liquid air was oxygen rich. Maybe even a teaspoon would be too dangerous, even with flack and hearing protection. Or maybe being so cold the
reaction less fierce?
I'd be extra cautious experimenting with liquid oxygen, you don't want to be a life after deflagration or detonation storyteller.
Propane freezing point
-306.4°F
-188°C
Flash point of propane −104°C (−155°F)
Liquid oxygen boiling point
-297.3°F
-183°C
[Edited on 21-12-2020 by Morgan] |
I don't know if it would explode. I would imagine it is as safe as having gaseous propane and oxygen mixture.
Also, I have heard that it is dangerous to spill LOX onto asphalt. It would explode. That information comes from a US military video information.
Also, it should be possible to freeze methane, ethane, butane.
methane =
-182.5 °C
-161.6 °C
ethane =
-181.76 °C
-89 °C
propane =
-187.6 °C
-42.09 °C
butane =
-138.4 °C
-0.5 °C
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Follow-up on asphalt ...
From Air Products
"Some of the organic materials that can react
violently with oxygen when ignited
by a spark or even a mechanical shock
are oil, grease, asphalt, kerosene, cloth,
tar, and dirt that may contain oil or
grease. If liquid oxygen spills on as-
phalt or other surfaces contaminated
with combustibles, do not walk on or
roll equipment over the area of the
spill. Keep sources of ignition away for
30 minutes after all frost or fog has
disappeared."
Sounds like asphalt is lively.
"If a vehicle drives over asphalt that has been impregnated by liquid oxygen, the impact of the tires on the oxygen-enriched asphalt causes a massive
explosion."
https://sciencing.com/spillage-asphalt-pavement-potentially-...
Liq O2 asphalt 1
https://youtube.com/watch?v=lY_BMzqP6rE
Oil and oxygen
https://youtube.com/watch?v=zFyqilT0ld0
What did I just watch
https://youtube.com/watch?v=MY5JbcjbgYE
[Edited on 22-12-2020 by Morgan]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Lol, this one is great!
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Tidbit
"In the past many physical properties of several air pollutants have been studied in mixture with air in process cryogenic conditions (from 90 to 150
K). Nevertheless for some of them, like ethane and propane, properties and behavior remain badly-known because of the difficulties inherent in the
handling of these reactive cryogenic mixtures. Only few solubility data of hydrocarbons in liquid oxygen have been published in scientific literature
and moreover appear as much dispersed according to authors. For example, literature brings back variations of experimental results of an order of
magnitude on the values of propane solubility in oxygen in cryogenic conditions."
"Solubility data of hydrocarbons in cryogenic liquids, and in particular in liquid oxygen will be very useful to design safe equipment for cryogenic
distillation of air into its constituents. The experimental study of the solubility of hydrocarbons in liquid oxygen is far from trivial due to
explosive properties of this type of systems."
https://www.aiche.org/conferences/aiche-annual-meeting/2007/...
|
|
|
EliasExperiments
Hazard to Self
 
Posts: 57
Registered: 3-3-2020
Member Is Offline
|
|
@Fulmen Thank you! Sure bigger rubber objects certainly would be a lot of fun. What do you mean with impact sensitivity?
@vmelkon I can try that, but what do you expect to happen? I don't think you can collect a usable quantity of mercury from that, because the amount in
there is around 1 mg
Testing LOX on asphalt sure would be interesting, but can I just buy regular asphalt? I need to see about that. Great idea! Sure I can freeze all
lower alkanes. I have tried it with butane once, it was not particulary spectacular. It just turned into a white solid, that melted very quickly. I
mean okay liquid methane is very impressive, if you burn it and pour it out. You get a nice dancing fire. But I am not sure where I can buy methane.
@Morgan Funny enough I already had the question how vigorously liquid propane reacts with liquid oxygen. I think I can do it safely. I mean with
explosives, especially if they are non toxic and you always keek your distance and use small amounts there is not a lot to worry about. Also if this
mixture fails to ignite I can just wait for it to evaporate and it would be safe. So I am most likely going to try that. 
The mixtures with oil and asphalt I am also going to try. Sounds like it is going to be a lot of fun. But that is only going to be possible in small
amounts.
Thank you for all the inspiration guys! As soon as I find a safe space where I can do the experiments you suggested, I will do them and then share
them here.
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
I would assume that any and all organic materials produce explosive compounds when mixed with LOX.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Explosions&Fire had a few things, benzene seemed on the cusp or something that will go bang big time. The Styrofoam brought to mind maybe
dissolving it down with acetone just to see how that would react, if lox would penetrate a slightly more condensed form or one of those clear
polystyrene cups. I guess it's partly how well the O2 mixes just as when I first experimented with KMnO4 and aluminum powder. The first mix burned
fast, the second better mixed went whoomp, and more finely powdered flat out exploded when a drop of glycerine placed upon an unconfined powdery pile
set it off. I had no idea I was working up to that outcome being a novice at the time. The more you know ..., I found a plastic Coke bottle cap filled
with the mixture and set off with a drop of glycerine makes a really perfect smoke ring along with the attention getting bang. I remember reading of
someone in a lab grinding a huge amount of permanganate and aluminum powder in a mortar and pestle and it blew a hole through the desk and he lost
body parts but lived. I shattered a small mortar and pestle doing the same by gently tapping the sides with the pestle to get the caking off the sides
when it happened. Not too smart but was unharmed. Then I took to using a brush for that and body protection and making sure the mortar was perfectly
clean of other substances.
Seems like a lot of lox reactions albeit perky, are burning at the boundary of two reactants, like the IPA and lox demonstrated. Are there lox
surfactants I wonder? Would methanol work any better than IPA? So many trivial questions.
Liquid Oxygen and Ozone
https://youtube.com/watch?v=szgejoiEXP0
[Edited on 23-12-2020 by Morgan]
|
|
|