EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Advice on safety for aldol products
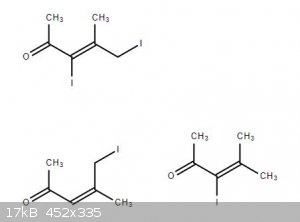
So I was planning on doing an aldol of acetone and iodo-acetone, however I cannot find any safety information from any chemical library, sigma aldrich
or other suppliers on these potential aldol products. There is only data for the 3-iodo-4-methyl-pent-3-en-2-one with a BP of 237C and a VP of
0+-0.5mmHg.
In the way of safety I plan on a small scale test of maybe 1ml of each reactant to prevent large issues, butyl rubber gloves, eyewear, and appropriate
lab clothing. But I wanted to get some other opinions. If anyone else thinks that this cannot be done safely because of the lack of information on the
products then I'm more than happy to not start the project. (Please note that I do not have a fume hood, but do not expect these products to be very
volatile due to the iodo groups.)
3-iodo-4-methyl-pent-3-en-2-one source https://www.chemspider.com/Chemical-Structure.73967163.html?rid=907ac7a4-af4f-42f0-b7fd-56fae985c192
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Isn't iodoacetone a lachrymator? Or is it too low-boiling?
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
Isn't iodoacetone a lachrymator? Or is it too low-boiling?
|
I have not come across any mention of iodoacetone being a lachrymator. Looking at the data it has a BP of 163C and a vapor pressure of 2.1mmHg.
Compared to Chloroacetone we know is a lachrymator with a BP of 119C and VP of 11.25mmHg. So I don't know how the volatility difference of 9.15mmHg
will alter the ability of it to behave as a lachrymator.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Science Madness is not a bad resource when it comes to chemistry related info. Check this: http://www.sciencemadness.org/talk/viewthread.php?tid=18579

A somewhat related info might be that iodoaceticacid-(m)ethyl ester is a decent lacrymator too.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Iodoketones are in general also lachrymators if the corresponding chloro- or bromoketones have lachrymator action.
But usually much less due to lower vapor pressure, they are easier to handle, usually.
|
|
|