ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
How reactive are gallium and indium in the reactivity series?
Hello. I was wondering just how reactive are gallium and indium in the reactivity series. Is it more reactive than tin?

The metal reactivity series.

"Imagination is more important than knowledge" ~Einstein
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
In general, elements in the same family (vertical column) of the periodic table, which you might enjoy learning about, have similar chemical
properties because they have the same number of valence electrons.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
https://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page)
This has everything!
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
You do have variations of electrode potential in group as well:
B(OH)3(aq) + 3 H+ + 3 e− ⇌ B(s) + 3 H2O −0.89
Al3+ + 3 e− ⇌ Al(s) −1.662
Ga3+ + 3 e− ⇌ Ga(s) −0.53
In3+ + 3 e− ⇌ In(s) −0.34
Tl+ + e− ⇌ Tl(s) −0.34
for the comparison:
Fe2+ + 2 e− ⇌ Fe(s) −0.44
Ni2+ + 2 e− ⇌ Ni(s) −0.25
Sn2+ + 2 e− ⇌ Sn(s) −0.13
On that page, though. several elements are missing for strictly comparable conditions.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Hah! I did the classic newbie error. You raised the question about the "reactivity series" and I immediately dived for the Activity
series.
Not the same thing. Let me explain.
The graphic you showed compares chemical reactions of metals and ranks them according to how vigorous the reactions are – roughly ranking them by
reaction rate which is a kinetic thing. A few common but arbitrary reactions are used to determine the rank.
But it is not hard to see that you could come up with a different ranking for reactivity by selecting different reactions. Reaction with chlorine gas
for example. Or maybe ethanol.
The point is that it is not a particularly rigorous test and therefore not a particularly helpful system for ranking metals.
The Activity series on the other hand is very useful. The list seems almost identical at first glance. But then you note the positions of calcium
and lithium and realise that something quite different is going on. Its better name is the table of standard reduction potentials.
It is not a ranking of metals, but rather a ranking of a very particular reduction reaction of the metallic ions. In other
words, not even considering other reactants, but just the movement of electrons.
It is an ordered list of reactions and not an ordered list of reactants. (Subtle and I repeat myself but this is significant.)
The table is quantitative and not merely qualitative.
The table is a measure of reaction potential. Therefore it relates to the energy of reactions and not the rate (which can be highly variable
depending on conditions.)
The table considers ther ionic species and not just the most common one that occurs in reactions with water and air.
The table extends naturally to non-metallic elements and other species as well.
So, although this little poster might look good on the wall of the high school lab, it really does not contain information that is that useful.
|
|
|
Syn the Sizer
National Hazard
   
Posts: 600
Registered: 12-11-2019
Location: Canada
Member Is Offline
|
|
The reactivity of an element is related to it's electronegativity is it not? You can tell how reactive an element is by chheckingb the
electronegativity table can't you?
https://www.ptable.com/#Property/Electronegativity
Fluorine is most reactive with an electronegativity of 3.98 and Francium least with an electronegativity of 0.70. All others fall in between.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
To say francium actually exists in any kind of practical sense is a bit of a stretch. But it can be expected to be similar to caesium.
And no. I don't think you could say that caesium is unreactive.
For sure, there are trends for all of these things in the periodic table. And therefore some correlation between them. But "reactivity", as in the
propensity to undergo chemical reactions, is probably the most vague and most difficult thing to nail down.
Is gold more or less reactive than carbon?
Is lead more or less reactive than selenium?
Is sodium more or less reactive than chlorine?
What about rhenuim (which does not react with aqua regia) and platinum (which does not react with hypochlorite)?
And what about red phosphorus and white phosphorus? Clearly there is a lot more to consider than just what element it is.
My thoughts are that Andy Brunning's chart (above) is somewhat useful for getting familiar with the properties of some elements. But for any
meaningful comparison between elements we need a more rigorously defined metric.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
By reactivity I mean the property of a more reactive metal precipitating a less reactive metal in pure form from its salts. Zinc chloride reacts with
aluminum in anhydrous conditions under heat to produce aluminum chloride and zinc metal, so aluminum is more reactive than zinc. Silver nitrate and
copper react to form a precipitate of silver metal and copper nitrate solution, so copper is more reactive than silver.
So the less electronegative element will precipitate the more electronegative element? So zinc and indium chloride or indium nitrate or indium sulfate
will make zinc salts and indium metal? So zinc and gallium sulfate reacts to form gallium metal and zinc sulfate? How about gallium sulfate reacting
with iron. Or iron sulfate and gallium reaction.
So going from more reactive to less reactive, is it zinc, indium, gallium, and iron, nickel, tin?
Is it zinc, iron, indium, gallium, nickel, tin?
[Edited on 21-7-2020 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by ChemistryGhost  | By reactivity I mean the property of a more reactive metal precipitating a less reactive metal in pure form from its salts.
[Edited on 21-7-2020 by ChemistryGhost] |
Which means you are comparing the elements according to their reduction potential (or oxidation potential which amounts to the same thing.) The
wikipedia page I cited is the thing you need.
The graphic in the OP is actually showing something quite different and the order of the elements is not the same. Lithium is in the wrong position
for example.
A couple of other things to note about the table of reduction potentials...
It ranks reactions rather than metals. Therefore it can give information on transition between Fe, Fe2+ and Fe3+ for example.
The Wikipedia page usefully has the two reactions related to water in bold. This means that all the reactions between these two positions are
reactions that can be done in aqueous solution under standard conditions. Anything outside that zone on the list are reactions that require
non-standard conditions such as molten salt electrolysis.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Table of reduction potentials.
So gallium will react with iron sulfate and make gallium sulfate and precipitate iron metal? Iron will react with indium sulfate to produce iron
sulfate and indium metal? Gallium will react with indium sulfate or indium chloride to produce gallium sulfate or gallium chloride and indium metal?
And both gallium and indium are more reactive than nickel or tin? In the gallium beating heart reaction, gallium sulfate reacts with iron to form iron
sulfate and gallium. But does that mean that gallium is the only one they got wrong on the table of reduction potentials? Or are there still some
reaction where gallium precipitates iron metal? I posted the table of reduction potentials.

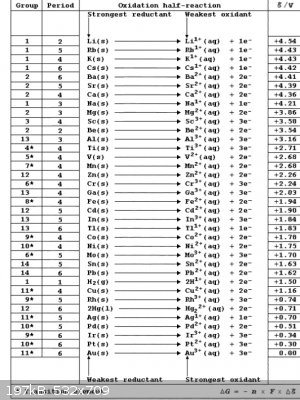
"Imagination is more important than knowledge" ~Einstein
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
That table of yours is strange. I have never seen Au used as the reference point for comparing potentials. H+ to H2 is the standard reference point
used. I would not vouich for all the information it contains. That said, you appear to have pretty much the correct interpretation of things. With
one important caveat.
Iron is weird.
It does have some reduction reactions with potentials that fall within the range of aqueous chemistry. But it is never reduced this way in practice.
At least I have never heard of industry producing iron by electrolysis of aqueous solution. The process usually requires blast furnaces and high
temperature carbothermal reduction and slag chemistry and so forth.
Iron produces a range of oxides and hydroxides and complexes that are favoured over the formation of Fe(s) in the presence of water. So while the
table may suggest Ga replaces Fe2+ with the formation of Fe metal, I don't think this happens in practice.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Quote: Originally posted by ChemistryGhost  | By reactivity I mean the property of a more reactive metal precipitating a less reactive metal in pure form from its salts.
[Edited on 21-7-2020 by ChemistryGhost] |
Which means you are comparing the elements according to their reduction potential (or oxidation potential which amounts to the same thing.) The
wikipedia page I cited is the thing you need.
The graphic in the OP is actually showing something quite different and the order of the elements is not the same. Lithium is in the wrong position
for example.
A couple of other things to note about the table of reduction potentials...
It ranks reactions rather than metals. Therefore it can give information on transition between Fe, Fe2+ and Fe3+ for example.
The Wikipedia page usefully has the two reactions related to water in bold. This means that all the reactions between these two positions are
reactions that can be done in aqueous solution under standard conditions. Anything outside that zone on the list are reactions that require
non-standard conditions such as molten salt electrolysis. |
No.
What it gives is the equilibrium allowed reactions with aqueous ions.
For example, consider equilibrium between lithium and sodium.
You could get a small amount of energy by reacting metal lithium with aqueous solution of sodium salt and precipitating metal sodium out of aqueous
solution.
But that reaction won´t happen, because the much faster reaction is reaction of metal lithium with water.
The order of reactivity is also not generally exactly the same in dry salt as in aqueous solution. If you react dry lithium chloride with sodium
metal, the equilibrium might be on the side of sodium chloride and lithium metal but lithium chloride might have the bigger heat of hydration.
On the other end, hydrogen peroxide and permanganic acid both release a lot of energy by evolving oxygen. Yet they exist, and function as strong
oxidants.
It is likely that iron is difficult to precipitate out of solution - because any reducer strong enough to reduce iron would reduce hydrogen rather
than iron.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
So the table of reduction potentials happens without electrolysis? And the metal that's a stronger reductant precipitates the weaker reductant metal
from it's salts? With the exception of iron with iron being higher than gallium. If so than ok. So the metals would be like in the table of reduction
potentials except that gallium is lower while iron is higher. So gallium would be more reactive than cadmium. And indium would be more reactive than
tin. And nickel is more reactive than molybdenum.
[Edited on 25-7-2020 by ChemistryGhost]
[Edited on 25-7-2020 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
It´s pretty much the same conditions as electrolysis. Basically, battery and electrolysis at the same time.
Quote: Originally posted by ChemistryGhost  | | And the metal that's a stronger reductant precipitates the weaker reductant metal from it's salts? With the exception of iron with iron being higher
than gallium. If so than ok. So the metals would be like in the table of reduction potentials except that gallium is lower while iron is higher.
|
No exceptions here.
Iron is hard to precipitate from aqueous solution, whether by electrolysis or by just putting a stronger reductant metal in, for the same reason that
sodium and all other strong reductant metals are hard to precipitate. The cathode - whether it is charged by external voltage or being the more
reactive metal that itself provides the voltage - if it is negative enough to reduce iron, it will reduce hydrogen instead.
For the same reason, it should be hard to precipitate gallium with a more reactive metal such as zinc or chromium.
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
i dun think
so, Ga is liquid at slightly higher temp so it can form as a liquid blob.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 563
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
The liquid state of Ga should not in itself favour its reduction over that of hydrogen, any more than the liquid state of Cs does.
But what may be relevant is the hydrogen overvoltages at various surfaces. For example, a Pt cathode has a strong tendency to evolve H2, while Hg
cathode is notably resistant to doing so, and even reduces alkali metals rather than hydrogen.
How prone is a liquid gallium cathode to evolve hydrogen from water?
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
So the table of reduction potentials is correct? Then how come iron reacts with gallium sulfate to produce gallium metal?
Here is silver precipitating from silver nitrate and copper.
https://youtu.be/1NKI0gxbQZA
Here is gallium sulfate reacting with iron to produce gallium metal and iron sulfate. Gallium precipitate.
https://youtu.be/g8Tc-5pUbH8
[Edited on 26-7-2020 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|