Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Preparing a chromatography column
I have never used column chromatography before but following a recent experiment in which I tried the benzal acetone reaction with salicyladehyde and
acetone I have run out of alternatives. The reaction gave a mixture of a pale green and a reddish brown crystalline products. I have managed to
manually pick out some of the pale green material and recrystallise it separately to obtain fairly pure pale green crystals but now the residue always
gives intergrown crystals that can't be hand picked. I have tried numerous solvents but their solubilitys are very similar so I thought I would try
column chromatography on some of the mixture. I have several columns with glassy frits in the bottom and taps below and I have a large tub of
chromatography grade silica gel.
The question is how do you actually construct the column? How do you stop the silica from washing through the frit (its a very coarse frit) and how do
you prevent bubbles forming in it? Has any one got any first hand experience and can point me in the right direction.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
How fine is your silica gel? you shouldn't need to worry about it passing through the frit if you're using the normal kind used for column
chromatography (70-230 mesh).
As for packing the column:
Close the stopcock and attach a funnel to the top of the column
OPTIONAL: Pre-fill the column with some solvent (I prefer not to do this but some people like to do it this way).
Prepare a slurry with a suitable quantity of silica gel and small amount of solvent (starting mobile phase)
Stir to suspend the silica and quickly dump the slurry all at once into the column
Rinse the walls of the column with solvent
Tap the column if necessary to dislodge any bubbles and settle the silica
Ensure the silica top is flat and level and top it with some acid-washed sand (optional) to avoid accidentally disturbing the silica gel later
Open the stopcock to run the solvent down to just at the top of the silica gel
Then you're ready to load the column and elute. Make sure not to let air be drawn into the silica gel bed because when you add more solvent it doesn't
effectively get rid of the trapped air and your separation might go to shit.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
there are a few ways to pack a column, i would suggest the wet method.
mix your silica with enough solvent (keep track of hoe much you are adding)to form a thin slurry (if you stop mixing it settles so be quick when
pouring), close the stopcock and pour the slurry down the column (be quick so the silica settles uniformly), wait for it to settle on its own and then
use a soft rubber tube to gently hit the column to pack the silica a bit more.
drain the solvent until the meniscus is right at the end of the silica column (never go below), keep note of the volume of solvent you get, the volume
of solvent you added to form the slurry minus the solvent you got now gives you the amount of solvent held in the column (when you later elute your
compound you can ignore this volume of solvent eluting through the column at the beginning as it won't have any of your compounds).
add your compound mixture dissolved in a minimal amount of the same solvent you are using as eluent, open the stopcock and again lower the meniscus
at the level of the silica (you can do this multiple times), now add the solvent slowly from the wall of the column to not disturb the top layer (you
can also add a layer of sand on top of the silica to protect it from the solvent falling in the column). you can start the elution process
there are many youtube videos that show you the packing process
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@DavidJR, many thanks for this info. Yes my silica is Silica gel 60 and 70-230 mesh. I haven't decided on solvents yet I am running some tlc first to
see what sort of solvent will be best.
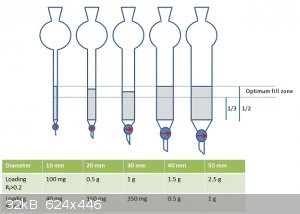
My column has an ID of about 16mm at a guess any idea what sort of loading I can apply in terms of how much of my mixture I can treat in one pass?
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
It does depend on how good a separation you get though. If well resolved you might be able to exceed the loading on Ubya's graphic by a considerable
margin.
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Could a burette be used as column? Or should i buy one?
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
You can do it in a syringe or pipette too.
the only limitation is the ratio of silicagel:product quantites. In my memeories you need 20 to 50 times more silicagel than product + impurities
I'm French so excuse my language
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
a burette is pretty thin and long, a normal 10ml pipette should have a similar diameter, but it's way shorter.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Eddie Current
Hazard to Self
 
Posts: 78
Registered: 25-7-2018
Member Is Offline
|
|
No. You will only end up blocking the burette making it useless.
Burettes are typically utilised for titrations.
You need to read/watch more video's of chromatography to gain a better understanding.
|
|
|
Housane
Hazard to Others
  
Posts: 127
Registered: 3-9-2018
Location: Worcester, England
Member Is Offline
Mood: Let’s make
|
|
Yeah I know what a burett is used for his seeing if I could use it for another thing @Eddie Current
Green QD's so far
Feel free to correct grammar or incorect knknowledge. We are all learning.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Eddie Current  |
No. You will only end up blocking the burette making it useless.
Burettes are typically utilised for titrations.
You need to read/watch more video's of chromatography to gain a better understanding. |
Why would it block up if you use glass wool and sand to keep the packing out of the valve?
I believe this was done on old columns that lacked a frit.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I have certainly read of burettes being used as columns. (See, for example, https://pubs.acs.org/doi/full/10.1021%2Facs.jchemed.8b00665)
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I think it more depends on the size of the silica used and not the burette/column.
If you have a very fine silicagel, like TLC silicagel 15µm, it might not work properly and get blocked.
|
|
|