ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
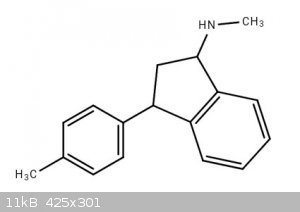
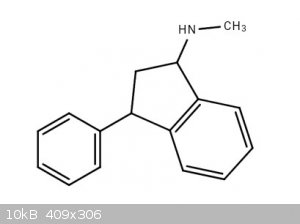
Synthesis of indatraline and analogs
Hello. I was wondering about the synthesis of indatraline and analogs of indatraline.
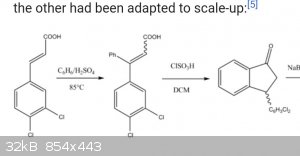
Starting from cinnamic acid. Can refluxing cinnamic acid with toluene result in addition like the aromatic phenyl part adds to the cinnamic acid, or
will the double bond react with the methyl group of toluene?
Starting from cinnamic acid. Will refluxing benzene add to the double bond?
Are there other ways cyclization can be achieved besides chlorosulfuric acid?
For the final step, I think the ketone can go through reductive amination with methylamine using aluminum galinstan. Sodium acetoxyborohydride can
also be used I think.
Indatraline




"Imagination is more important than knowledge" ~Einstein
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I guess that toluene reacts in para position because sulfuric acid forms p-toluenesulfonic acid and sulfonic group is the reactive one there.
It's hard for me to tell if benzene would undergo the same reaction as I don't know differences in rectivity between toluene and benzene sulfonic
acid.
If I'm wrong let anybody tell me.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|

Indanetraline.

4-Methylindanetraline
"Imagination is more important than knowledge" ~Einstein
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
A link to the source of that benzene/sulfuric acid reaction, or at least the Wikipedia page you got that image from would have been helpful. I'm pretty sure the
lack of either is the reason you got no replies. Also cutting the image in half doesn't help, especially not when part of your question refers to the
part you cut... wouldn't a link to the whole image have been more informative and even easier on your side?
But, yes, I'm pretty sure this reaction would run with toluene, giving the para methyl phenyl product. The benzene reaction goes via benzenesulfonic
acid. With toluene para-toluenesulfonic acid is formed. There is no way the toluene methyl group would react with the double bond.
[Edited on 2-6-2020 by Tsjerk]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
[Edited on 6/6/2020 by Yttrium2]
|
|
|