Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Success: Pressure from pirani gauge: Inficon PSG500
Up to now I've been measuring pressure in the lab using a classic manometer ("Bourdon gauge"). While it is reliable and cheap, it is only useful down
to about 95 % vacuum (50 mbar). Below that, the needle moves too little to give any information. In addition, a manometer like this measures pressure
relative to atomspheric pressure, so even atmospheric variations due to weather (50 mbar swings) are greater than the absolute pressures we might be
interested in.
There are times where knowing the pressure down to mbar level and below is useful (vacuum distillation, etc.), where a Bourdon gauge is not
applicable.
To remediate this, I got a Pirani gauge on ebay. Pirani gauges are typically used from 10-3 to 103 mbar. The one I got is a
PSG500 made by Inficon, but other manfacturers exist. New, they cost about $500 but you can get one on ebay for about $100. Now these usually require
a "Pirani gauge controller" to operate, but in the case of the PSG500, all you have to do is supply 14-30 V, and read the pressure as a 0-10.3 volt
signal. The signal U [volts] is analog: a simple logarithmic function of the pressure: p[mbar] = 10^((U - 6.143)/1.286)
Connections are made using an RJ45 cable ("ethernet cable", 8 conductors, $5), which can be butchered (carefully) to get access to individual wires.
The pinout is given on page 3 of the manual attached below.
I hooked up my gauge to my KNF membrane vacuum pump to test it, and got a voltage of 7.5 V, which corresponds to about 11 mbar. It works! I'll
probably make a proper control circuit with an analog voltmeter as readout, at which point I'll report here.
This post is just to report my success, in case anyone wants to mess with this model of pirani gauge.
Bourdon gauge:

Pirani gauge KF-16 flange side + accessories:

Pirani gauge RJ45 connector side + cable:

Operating manual:
Attachment: Inficon PSG500 operating manual.pdf (273kB)
This file has been downloaded 399 times
Pressure voltage curve:
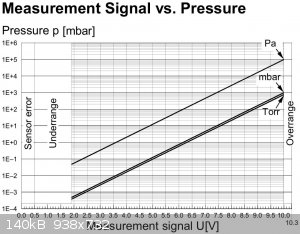
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Thank you for posting this. It's always useful to have this type of information to refer to. It's not every day when someone says, "Hey I just spent
money and here's the data that I gathered from it"!
So what is the first project that you plan to use this on?
Also, these gauges are not difficult to clean with an isopropanol rinse. I use one of them when pumping down refrigeration systems, and it readily
collect volatile oils along with other contaminates. A simple rinse is what it takes to clean it out.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I've been looking at some dual stage rotary vane pumps recently, hoping to reach 10-3 mbar with one for some electron beam experiments. An
electron beam melter/evaporator and possibly some x-ray experiments too. Electron beams and x-rays would also nicely complement my fascination for
phosphors. I also plan to distill sodium metal to make some ampoules for my collection. With my approx. 10 mbar pump, I can lower the boiling
temperature to 550 °C IIRC, which is low enough to conduct the distillation in borosilicate glass with a blowtorch (carefully) but still a bit
impractical. But at 10-3 mbar, the boiling point should be around 230 °C. At 10-1 mbar: 347 °C. These temperatures are easily
manageable with a heatgun even. Potassium, rubidium and cesium should be a breeze if sodium can be distilled.
I needed some form of vacuum gauge because I don't want to buy a pump without a way to test it. This Pirani gauge should just about cover the range of
pressures achievable with a dual stage pump, so I'll know if what I bought is up to specs or not. The manual says it can measure from
5x10-4 to 1000 mbar. I'd like to avoid using a turbomolecular or diffusion pump for now so I'll go with a really good rotary vane pump.
In addition, actually building a pirani gauge controller from scratch is really satisfying (even though in this case it happens to be trivial, the
signal processing is done inside the gauge itself).
Thanks for that info on cleaning! Do you have to disassemble it or do you just pour iPrOH directly through the port? Thinking about it, the gauge has
to be airtight so I assume it's safe to pour the liquid directly?
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
The gauge should just be a wire, sealed inside of a tube. Isopropyl alcohol is fine for it. Generally all these gauges need is a quick rinse and
gentle blow dry with dry air. I'm re-assembling an old FEI FIB, and that's basically all that I did with the Pirani gauges. My refrigeration micron
gauge also says to do this cleaning with isopropanol regularly.
Cold cathode gauges are a different story. These work at higher vacuums and voltages. Pump oil vapors will slowly foul them, and these will
generally need to be taken completely apart and cleaned of all the built-up carbonaceous crud.
It sounds like you have some interesting projects lined up. I hope you find time to post them here.
In the back of my mind I am still tossing around the idea of crafting a fully-developed writeup on a benchtop Ostwald Process. Additionally, I'd like
to tackle the Haber Process in the same way. I have no spare time right now, though.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Ah yes, the Ostwald process! Have you had any developments in the last year? You project looked well on its way to be successful, especially with your
very nice catalyst support ideas!
My own reactor has been improving, but very slowly. I have constructed a packed gas absorption column for the nitrogen oxides: Packing is Raschig
rings, and water/HNO3 is circulated on the packing with an Iwaki type chemically resistant pump. The whole thing is over 1.3 meter tall and very
satisfying to see in action, but I haven't tested it with nitrogen oxides properly yet.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I’ve put a lot of thought into it, but haven’t done any more actual work on the reactor since I posted my progress here. I bought a pound of
Veegum-T to use as a super-plasticizer for the catalyst support, since I would like to bump up the alumina concentration from 90% up to about 95-99%.
The Veegum-T is a much stronger plasticizer than bentonite clay, and has much lower iron content. This much Veegum-T is essentially a lifetime supply
for me.
Instead of using an aluminum plate as a mold like before, and then having to dissolve the mold away with acid to free up the catalyst support, I’m
thinking of replacing the aluminum with wax. Some gentle heat could be used to hopefully melt the mold while at the same time soaking the green
ceramic with wax for easier handling. As with anything else, I have to actually try this idea to see if it would work without crumbling the delicate
clay body.
I seldom work on one project at a time. Another reason that I’m so interested in developing better ways of fabricating small alumina ceramics, is
that I’m trying to build my own gallium sources for the FIB. These seemingly simple things are very expensive if you just buy them. This task
involves a mix of ceramics, welding, and some difficult manipulations in high vacuum. I’m trying to develop an easier way.
It sounds like your reactor is more practical than mine generally. I’m trying to compress my Ostwald reactor down into something very small and
portable. I would also like to get close to 100% conversion of the nitrogen oxides to nitric acid. Also, I’m thinking to go directly to fuming
nitric. Right now this uses liquid nitrogen to condense out the N2O4 (it actually freezes it out, creating a problem with clogs), but perhaps I can
develop a more efficient condenser that operates at a higher temperature. Pure oxygen and small amounts of water would be fed into the reaction to
create the fuming nitric acid directly. If you have the space, though, an absorption tower is the simplest way to go for creating diluted acid.
I’d enjoy seeing what you have going once you’re ready.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Heptylene  | | I also plan to distill sodium metal to make some ampoules for my collection. With my approx. 10 mbar pump, I can lower the boiling temperature to 550
°C IIRC, which is low enough to conduct the distillation in borosilicate glass with a blowtorch (carefully) but still a bit impractical. But at
10-3 mbar, the boiling point should be around 230 °C. At 10-1 mbar: 347 °C. These temperatures are easily manageable with a
heatgun even. Potassium, rubidium and cesium should be a breeze if sodium can be distilled. |
Please record a video when you do this. It sounds really interesting -- I've never seen solid metals distilled in glassware. 
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by monolithic  | Quote: Originally posted by Heptylene  | | I also plan to distill sodium metal to make some ampoules for my collection. With my approx. 10 mbar pump, I can lower the boiling temperature to 550
°C IIRC, which is low enough to conduct the distillation in borosilicate glass with a blowtorch (carefully) but still a bit impractical. But at
10-3 mbar, the boiling point should be around 230 °C. At 10-1 mbar: 347 °C. These temperatures are easily manageable with a
heatgun even. Potassium, rubidium and cesium should be a breeze if sodium can be distilled. |
Please record a video when you do this. It sounds really interesting -- I've never seen solid metals distilled in glassware.  |
Here's one of rubidium being distilled (not my video):https://www.youtube.com/watch?v=Ur7LW7fgbsU
And a photo of my test tube scale sodium distillation attempt. The little spheres are sodium condensation droplets. For reference, test tube is 18 mm
diameter. Sodium vapor is a lovely shade of green, really pretty! I don't have any picture of the vapor though.

|
|
|
|