fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
What's the minimum concentration for h2so4 to be oxidising?
What's the minimum concentration for h2so4 to be oxidising? (rxn to form SO2)
I can't find this info.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Depends on reducing agent, temperature, etc. Chemistry.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Sorry, just to tack on this thread ... what about the concentration to be dehydrating?
For example to dry liquid bromine or chlorine/SO2 gas? 80% ok?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by artemov  | Sorry, just to tack on this thread ... what about the concentration to be dehydrating?
For example to dry liquid bromine or chlorine/SO2 gas? 80% ok? |
I guess somewhere around 37%, according to the graph attached, but how dehydrating do you want it?
[Edited on 6-11-2019 by Tsjerk]
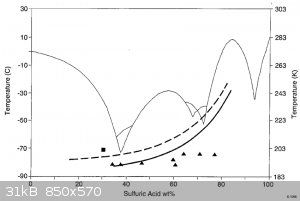
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
To my experience, sulphuric acid starts to become somewhat oxidizing at a concentration of 60 to 65% by weight. If I mix equal volumes of water and
97% H2SO4 (the latter has 1.84 times the density of water) and I add this mix to e.g. KBr, then a little amount of Br2 is formed, while at 40% no Br2
is formed. Temperature has a strong influence. If the acid is warm, then more Br2 is formed.
Dehydrating power most likely requires higher concentration.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Quote: Originally posted by woelen  | To my experience, sulphuric acid starts to become somewhat oxidizing at a concentration of 60 to 65% by weight. If I mix equal volumes of water and
97% H2SO4 (the latter has 1.84 times the density of water) and I add this mix to e.g. KBr, then a little amount of Br2 is formed, while at 40% no Br2
is formed. Temperature has a strong influence. If the acid is warm, then more Br2 is formed.
Dehydrating power most likely requires higher concentration. |
Have you done the same for iodide?
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
What about rxn with Cu?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Or with Hg, per comment:
"Concentrated sulphuric acid possesses marked oxidising power, especially in the presence of certain metallic salts, such as those of mercury and
copper."
See comments at http://sulphur.atomistry.com/sulphuric_acid.html .
Sample reaction with concentrated acid:
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Source: https://chemiday.com/en/reaction/3-1-0-270
[Edited on 16-11-2019 by AJKOER]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Sigh. My question is the min conc for H2SO4 to be able to oxidise Cu.
|
|
|
vibbzlab
Hazard to Others
  
Posts: 241
Registered: 6-11-2019
Member Is Offline
Mood: Always curious
|
|
Do copper metal react in concentrated Sulfuric acid. I actually never tried that 
Amateur chemist. Doctor by profession
Have a small cute home chemistry lab.

Please do check out my lab in YouTube link below
This is my YouTube channel |
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
Yes it does react, at least when the sulfuric acid is heated. I saw a small blue coloration even without heating it, but I'm pretty shure, that this
is just because of the oxide layer on the copper
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
For oxidation of copper metal you need highly concentrated acid (90+%) and even then, some gentle heating is necessary as well.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | | For oxidation of copper metal you need highly concentrated acid (90+%) and even then, some gentle heating is necessary as well.
|
which I find very interesting given an alternate path that I have presented that does introduce, however, a sodium ion presence. A partial quote from
https://www.sciencemadness.org/whisper/viewthread.php?tid=15... :
Quote: Originally posted by AJKOER  |
.........................
Employing NaHSO4 as the source of the bisulfate, the net reaction would be:
Cu + H2O2 + 2 NaHSO4 ---Activated Carbon, Microwave---> CuSO4 + Na2SO4 + 2 H2O
where the expected k > 10^8 (based on p. 15 reported reactions by •SO4- acting on Fe(ll), Ce(lll), Cr(ll),.. at https://srd.nist.gov/NSRDS/NSRDS-NBS-65.pdf by Ross and Neta)
[Edited on 5-7-2019 by AJKOER] |
However, the science I outlined in the referenced thread, is based on a process to radicalize the -HSO4 ion (and this can also be accomplished with
even weak H2SO4 as a source of HSO4-). A relevant comment at https://www.sciencedirect.com/topics/earth-and-planetary-sci... :
"Sulfate radicals are one of the strongest oxidants available, with an oxidation potential of 2.6V, as compared to the hydroxyl radical (2.7V), the
permanganate ion (1.4V), and ozone (2.2V) and are effective in oxidizing a broad range of chemical substances."
[Edited on 4-12-2019 by AJKOER]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I know I shouldn't comment on AJKOER's comments, but AJKOER... Have you tried to perform the reaction you propose while taking the proper controls in
account?
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tsjerk  | | I know I shouldn't comment on AJKOER's comments, but AJKOER... Have you tried to perform the reaction you propose while taking the proper controls in
account? |
Well, we can discuss what constitutes good 'controls', but I simply reply, let's start by examining the reaction pictures provided in the link, and
the amount of time involved to produce that degree of coloration.
I would also add that in the current context, the proposed reagents, in addition to pure copper metal, could include H2O2, dilute H2SO4 and a small
amount of pure CuSO4 (as an electrolyte, or as pre-formed by adding, for example, CuO, from heated copper metal, or Cu(OH)2 or CuCO3 to H2SO4). The
choice of the activated carbon source is a selection based on several factors (like accessibility, cost, purity requirements, review of the
literature,....). High purity graphite rods (used in mechanical pencils) are likely also suitable here, perhaps crushed to increase surface area.
[Edited on 4-12-2019 by AJKOER]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Just found an interesting but old text accounting for the products of the action of H2SO4 on Cu, which can be a problematic reaction apparently due to
a sulfide coating on the copper metal (alleviated by heating the reaction mix) and the evolution of SO2 at https://books.google.com/books?id=9SdZxFuyYGAC&pg=PA112&... .
For those seeking standard chemistry, apparently not so, with the proclaimed liberation of nascent hydrogen (more correctly the hydrogen atom or
radical). This interestingly parallels the surface formation of .H cited by the action of Zn with HCl. However, in the latter case, there is no
apparent action of .H on HCl as:
.H <--> H+ + e-
.H + H+ + Cl- = H+ + e- + H+ + Cl- = HCl + .H
[Edited on 5-12-2019 by AJKOER]
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
Is the nascent Hydrogen made manifest via the medium of the aether?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So my thinking here is, molecular H2SO4 is oxidizing, but HSO4- and H3O+ are not. We want to know the critical concentration of dihydrogen sulfate at
which [H2SO4] is not very small. Since H2SO4 is strong in water, it’s probably close to (and definitely less than) a 1:1 molar ratio of H2SO4:water.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Indeed, H2SO4 is oxidizing, HSO4(-) is much less so and SO4(2-) is not. You need concentrations of 85+ % of H2SO4 in order to have reasonable amounts
of H2SO4, otherwise you have mostly HSO4(-), which is less oxidizing. The latter, however, is not completely free of oxidizing properties. Mix a
little KBr and NaHSO4 and heat in a test tube. You get mostly HBr, but also some Br2.
This behavior is quite common with oxo acids. A similar observation can be made with HNO3 and NO3(-), somewhat more pronounced than with H2SO4. Much
more pronounced do we observe this with HClO4 and ClO4(-). Anhydrous HClO4 is insanely dangerous and reacts nearly explosively with many reductors
(e.g. paper, sulphur, sugar, fabric, wood), while ClO4(-) is amazingly inert in aqueous solution (e.g. 70% acid does not react with iodide ion or with
SO2 and when zinc metal or even magnesium metal is added to such acid you only get H2 and no reduction of ClO4(-)).
The reason behind the difference in oxidizing power between the molecular acid and the ionized acid is that in the ion, there is good symmetry and
resonance stabilization. E.g. the outer electrons in ClO4(-) are equally distributed over all four oxygens. Similar stabilization also occurs in
nitrate ion and sulfate ion. Salts of these acids, therefore also are stable. Sulfates, nitrates and perchlorates are stable salts for all metals and
many, even strongly reducing, organic cations. On the other hand, esters of these acids, especially the nitrates and perchlorates, are very dangerous
and can be made to explode by simply looking angry at them 
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Here is a more modern rendition, to quote a comment from a demonstration on 'Electrochemical Catalyst' supplied by Jeff Hughes at Applied Chemistry
Department, RMIT at http://chem.lapeer.org/Chem1Docs/ElectChemCat.html which looks at the reaction between zinc metal and dilute sulfuric acid, without and with the
presence copper metal (created in the cell), the latter promoting a favorable formation of an electrochemical cell.
Further, as to mechanics, the comment:
"The reason for the differences in overpotentials is related to the ease of formation and evolution of H2 bubbles, which occurs in several steps:- (i)
diffusion of H+ to the metal surface; (ii) H+ + e => H; (iii) formation of a layer of M-H i.e. H atoms adsorbed on the metal surface; (iv) H +
H => H2; (v) formation and evolution of a bubble from the surface"
Per above:
Quote: Originally posted by AJKOER  |
.....................
I would also add that in the current context, the proposed reagents, in addition to pure copper metal, could include H2O2, dilute H2SO4 and a small
amount of pure CuSO4 (as an electrolyte, or as pre-formed by adding, for example, CuO, from heated copper metal, or Cu(OH)2 or CuCO3 to H2SO4). The
choice of the activated carbon source is a selection based on several factors (like accessibility, cost, purity requirements, review of the
literature,....). High purity graphite rods (used in mechanical pencils) are likely also suitable here, perhaps crushed to increase surface area.
|
And:
Quote: Originally posted by AJKOER  |
................
For those seeking standard chemistry, apparently not so, with the proclaimed liberation of nascent hydrogen (more correctly the hydrogen atom or
radical). This interestingly parallels the surface formation of .H cited by the action of Zn with HCl. However, in the latter case, there is no
apparent action of .H on HCl as:
.H <--> H+ + e-
.H + H+ + Cl- = H+ + e- + H+ + Cl- = HCl + .H
|
So, in my proposed reaction cell, copper and carbon are in an electrolyte likewise creating a beneficial electrochemical cell. Copper, the more
reactive metal, is the anode, carbon is the cathode, so hydrogen ions are likely to be reduced to hydrogen atoms at the carbon electrode and may
further react with H2O2 resulting in active radicals and associated products including CuSO4.
[Edited on 7-12-2019 by AJKOER]
|
|
|