wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Dichloro m-xylenol reactivity
Are the Cls in DCMX more reactive than the ones on p dichlorobenzene?
I am considering buying some DCMX and I am hoping that as it a phenol the Cls will be reasonably reactive meaning more reactive than PDB.
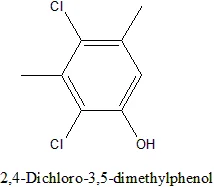
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
More reactive in which reaction? 
If you are thinking about nucleophile substitution then the answer is no. No because it'd require strong electron withdrawing groups to "activate" the
chlorines towards nucleophilic susbstitution. o-and p- substituents relative to the chlorine are required for such an effect, m-substituents have no
effect.
Moreover those methyl-groups may flank the chlorines nicely, further decreasing their reactivity.
You read my theory.
Now let's allow others to chime in! 
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Pumukli  | More reactive in which reaction? 
If you are thinking about nucleophile substitution then the answer is no. No because it'd require strong electron withdrawing groups to "activate" the
chlorines towards nucleophilic susbstitution. o-and p- substituents relative to the chlorine are required for such an effect, m-substituents have no
effect.
Moreover those methyl-groups may flank the chlorines nicely, further decreasing their reactivity.
You read my theory.
Now let's allow others to chime in! 
|
Hopefully its an easy route to various xylenol derivatives used in the synthesis of various triarylmethane dyes. Such as m-xylenol, substituting the
Cl p to the the OH with various amines and perhaps the Cl o to the OH with SO3H.
At about 14pounds for 500ml including delivery its cheap.
[Edited on 10/31/2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Pumukli
National Hazard
   
Posts: 705
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Your planned reactions have no chance to work on this substrate.
You would need either nitro or cyano or a few other, similarly strong electron withdrawing groups to make the chlorines reactive enough.
The methyl groups are not electron withdrawing, they are electron releasing, OH too.
Check the requirements of aromatic nucleophile substitution in the literature!
|
|
|