chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Effective antifoaming agents used with organic solvents
Can anyone recommend one?
Preferably it is unreactive, i.e. tolerated by acylations.
Solvents I'm thinking of are DMF, DCM, NMP, including non-nucleophilic bases.
Silicone polymers seem the material of choice, but there are so many, perhaps someone has some experience and/or advice?
For instance, what do you think of this stuff
http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en...
Sadly they don't give a structure - it'd help!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Where I worked in industry Dow defoamers were used successfully on paper pulp washers and in a brine evaporator.
This MSDS may help in determining the main ingredient:
http://www1.dowcorning.com/DataFiles/090007b281460737.pdf
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
THanks- but I'm more after an antifoamer that is already anhydrous, and explicitly useful for organic solvents.
The scenario is, a reaction vessel receives constant N2 bubbling, but at times it foams so much that reactants come out at the top end.
Easy, you'll say, just reduce the N2 flow- but that isn't easily done - plus the N2 is required to for mixing. So I thought an antifoaming agent might
be the smartest solution...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Mechanical - if your reaction setup involves ground glass joints, employ a foam breaker:
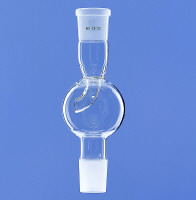
You can also reduce foaming by lowering surface tension of the liquid. I sometimes add dichloromethane, hexane or diethylether for rotary evaporation
of foamy solvents. But with N2 bubbling they'll be gone quite quickly.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Chemoleo, I wasn't so much thinking that the Dow product per se would be what you're looking for but the silicone name might be of use, eg,
polydimethylsiloxane, etc.
Here's one for the oil & gas industry that is specifically all organic:
http://www.dowcorning.com/applications/search/default.aspx?R...
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|