draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Need help finding lithium hydroxide in oz
Does anyone know where I can get lithium hydroxide in Australia? eBay was no help being too expensive or not available, Google was no help (unusual),
Alibaba will take ages with back and forth emails drawing up contracts etc etc, I can't find any soapmaking supplier that sells it.if anyone knows a
company that ships to Australia please share.
|
|
|
Keras
National Hazard
   
Posts: 901
Registered: 20-8-2018
Location: (48, 2)
Member Is Online
|
|
Err
This Aussie shop claims to sell lithium carbonate pills. You should be able to get lithium hydroxide from it.
Otherwise, try chemcraft.su? (see attached screenshot)
[Edited on 28-6-2019 by Keras]
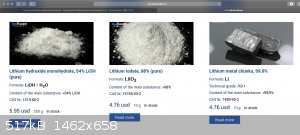
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Nilered said Li metal is cheaper than the hydroxide (I think in mole basis), so why not try buying Li metal?
|
|
|
Keras
National Hazard
   
Posts: 901
Registered: 20-8-2018
Location: (48, 2)
Member Is Online
|
|
Quote: Originally posted by fusso  | | Nilered said Li metal is cheaper than the hydroxide (I think in mole basis), so why not try buying Li metal? |
Well, if you look at the screenshot I posted above, 10 g Li is about the same price as 250 g LiOH…
|
|
|
j_sum1
Administrator
       
Posts: 6324
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I once found lithium hypochlorite as a pool chemical. My intention it to use it on chlorine generation and then later isolate a lithium compound from
the waste. Probably lithium carbonate: this is sufficiently insoluble as to make for a good precipitate.
Not sure if that helps, but it was OTC and will not be too complicated to do.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Yeah I remember seeing lithium something as a pool chemical once the hypochlorite could just be treated with HCl venting the cl gas and boiling dry to
yeild the chloride followed by washing and redissolving in distilled water and electrolyzed to the hydroxide.only thing is I don't want to dedicate a
day or two to just preparing the hydroxide.and I imagine I'd need carbon electrodes to not end up with a bunch of metal impurities from using a ss
electrode.As for starting from the metal to make the hydroxide would be pointless and expensive just to turn back into the metal ( if possible ).I'm
not into circular chemistry.well it's good for recycling plastic but pointless in this case.
|
|
|