Ze_alpha
Harmless

Posts: 1
Registered: 15-10-2010
Member Is Offline
Mood: No Mood
|
|
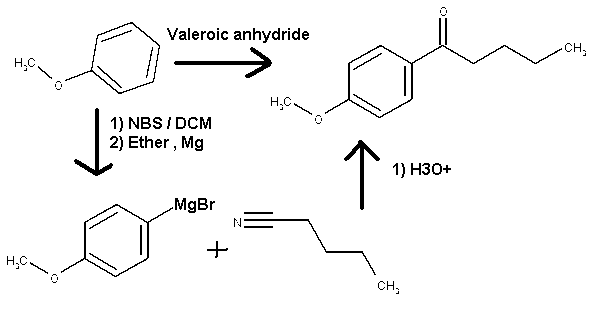
Pathway to acheived 4-methoxy-phenylvalerophenone from 1-methoxybenzene
Hi, Everybody
First of all this forum is really nice and keep on going you are really doing a great job.
I would like to ask you a question guys about a reaction. In one of my synthesis I want the chemical 4-methoxy-phenylvalerophenone .I'm a little bit
confused regarding the pathway to achieved my goal. My starting chemical is the 1-methoxybenzene.
The more logical pathway for me will be to do an electrophilic substitution on the aromatic ring with an excess of valeroic anhydride to yield
directly to the desired compound. I'm wondering if by extending the alkyl chain of the acid anhydride with more carbon there will be a lost in
reactivity. Is this can be predictable? The only ref I found is with propionic anhydride on wiki
My second logical pathway will be to brominate the 1-methoxybenzene with NBS, reacting the 4-methoxybromobenzene in the art to make the corresponding
Grignard and reacts it with pentanitrile to achieved the desired chemical. Here again I’m not able to find substainable pathway of reaction
regarding grinard on nitrile. Is it because the nitrile are not a good substrate for Grignard reaction in ethereal medium?
I know the diagram is wierd and sketchy, none exact but it's just to visualise all the thing
Thank you for your help !
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Well, I goofed pretty badly with that first attempt at a reply, so let me try this again!
Bromination with NBS should be fine. According to wiki*, using DMF as a solvent gives high para-selectivity. The rest of the scheme looks good as
well.
I think doing the Friedel-Crafts acylation with Valeroic anhydride would be the better of the two routes though. Fewer steps = less product loss.
However, the disadvantage of this route is that you do not get the para-selectivity, so your yield may only be ~33%. The longer alkyl chain should
not have an effect on the acylation.
*the article cited is Mitchell, R. H.; Lai, Y.H.; Williams, R. V. (1979). "N-Bromosuccinimide-dimethylformamide: a mild, selective nuclear
monobromination reagent for reactive aromatic compounds". J. Org. Chem. 44: 4733. The wiki page I'm referring to is http://en.wikipedia.org/wiki/N-Bromosuccinimide#Bromination_...
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
|