nelsonB
Hazard to Self
 
Posts: 71
Registered: 5-9-2013
Member Is Offline
Mood: No Mood
|
|
benzoic acid to bromobenzene
Hi
I was wondering what would be better in both of these pathway

decarboxylating benzoic acid to benzene then bromination to bromobenzene,
or
bromination of benzoic acid then decarboxylation to bromobenzene.
its seem trivial but sure one would be better than the other right ?
[Edited on 4-3-2019 by nelsonB]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Benzoic acid will be more difficult to brominate than benzene, because the carboxylic acid group is deactivating.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
But you'd also avoid making di- or tri- bromobenzenes by brominating benzoic acid, and it avoids dealing with benzene directly. You could also save
some of the m-bromobenzoic acid for other uses.
Edit: Then again, it may be harder to purify the bromobenzene from the decarboxylated gunk, since it has a much higher boiling point than benzene.
Fractionating it from the impurities would be less trivial.
[Edited on 3-4-2019 by Texium (zts16)]
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Think about the fact that if the decarboxylation has a 50 % yield, 50 % of your bromine will have been wasted into side-products. Whereas if you
brominate benzene, the yield will be much better, less bromine wasted.
That's why you should try to use the expensive reagents near the end of the synthesis.
Unless of course you can get bromine easily, then this isn't an issue.
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I would decarboxylate to benzene, and then brominate.
Or...
You can react the benzoic acid with PPA and nitromethane to get aniline via a Lossen Rearrangement. From there, Sandmeyer reaction with CuBr and poof!
Bromobenzene.
http://www.prepchem.com/synthesis-of-bromobenzene/
http://reag.paperplane.io/00002328.htm
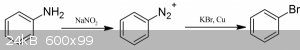
[Edited on 4-3-2019 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Does Hunsdiecker reaction work on benzoic?
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Here is a question on StackExchange Chemistry.
https://chemistry.stackexchange.com/questions/71706/aryl-hal...
| Quote: |
There might be more recent publications, but an old review by Robert G. Johnson and Robert K. Ingham, Chem. Rev., 1956, 56, 219-269, gives some
directions.
Based on early attempts with benzoic acid that date back to the time around 1840, is was long believed that the silver salts of aromatic carboxylic
acids do not undergo a Hunsdiecker reaction at all. The authors write:
In no case was bromobenzene obtained as a result of the action of bromine on silver benzoate. The product of Peligot and Bunge was a monobromobenzoic
acid; apparently Kekule’s experiment was completely unsuccessful.
However, it later turned out that the inital verdict wasn't quite true.
Benzoic acids bearing electron-withdrawing substituents, such as Cl or NO2 can be converted to their corresponding bromoarenes in a Hunsdiecker
reaction. The authors of the review denote:
Thus, the three isomeric nitrobenzoic acids (as silver salts) were converted to the corresponding bromides in excellent yields (ortho, 95 per cent;
meta, 89 per cent; para, 79 per cent). The yields from the chlorobenzoic acids were less satisfactory, but sufficiently high to indicate the efficacy
of the chloro group. |
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
How much Bromo-Benzene do you need?
Benzoic Acid is cheap and available.
Aniline is not cheap, and is less available. Though Acetanilide might be had, for a price. A positive is, that diazotization etc.... of aniline,
might produce only one product.
Benzene used to be cheap, but is now tough to obtain. Bother!
P-Aminobenzoic acid, is available, but not super cheap. Easily? decarboxylated to Aniline, in high yield I'm guessing.
Aniline becomes Bromobenzene fairly easily.
Two reaction steps, is seldom better than One step, but there might be mitigating circumstances.
https://www.amazon.com/BulkSupplements-Pure-Para-AminoBenzoi...
Pure bulk price. $33.50/Kg. https://purebulk.com/products/para-amino-benzoic-acid-paba-v...
Note: After I shot off my big mouth, I checked around for PABA Decarboxylation procedures.
Seems like it should be easy. Like making pyridine-easy.
However, No PABA procedures sprang to the forefront. So, maybe I was wrong..... Probably I wrong.
Billy Joel speaks for all of us?
https://www.youtube.com/watch?v=cih0btgJw8s
[Edited on 4-3-2019 by zed]
[Edited on 4-3-2019 by zed]
[Edited on 4-3-2019 by zed]
[Edited on 4-3-2019 by zed]
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Bromination of benzene is as easy as some steel wool, benzene, and bromine. Yields are easily >80% and dibromobenzene is left behind when
distilling the product. This seems like a lot of maneuvering if you were planning to use bromine anyway.
As for OP, definitely the second path. Decarboxylation in refluxing quinoline with copper catalysts works well for benzoic acids, but if your stuff
isn't valuable, it's not worth it. The alkali fusion with NaOH if cheap and effective but it may react with the brominated ring first and it produces
some degree of crud either way.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Brominating the benzoic acid first could lead to the br being replaced by the OH from the caustic leaving a useless phenol couldnt it like how
clorobenzene is converted to phenol.
[Edited on 5-3-2019 by draculic acid69]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
what about using this method - https://pubs.acs.org/doi/abs/10.1021/jo00167a027
|
|
|