EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
which is the better reflux condenser? allihn vs friedrich
from the little bit of info i've read it seems like the friedrich is the way to go. i have a 200mm allihn that serves me well for a myriad of
refluxing tasks but i am in need of a 400mm reflux condenser so i'm thinking of getting a friedrich. it will be used for refluxing a variety of
things from mild refluxing of random solvents to heavy vigorous refluxing of gas-laden solvents.
i've googled all last night and this morning and have a slight grasp on why the friedrich is better but am curious if any of the experts here have any
personal experience they wish to contribute to sway my decision one way or the other?
i'm also curious, why do so many people slam graham condensers as being nearly useless? from the diagram, they would appear to be the most efficient
at condensing vapors because of the long path the vapors have to travel while being surrounded by the coolant. certainly i'm missing some logic in
this.
as always, thanks
[Edited on 30-7-2010 by EmmisonJ]
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
Which ever one works best for your application.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Similar to a Fredrich is a jacketed coil condenser. It depends though on what you want to reflux and why, i.e., if you're going to be off-gassing
during the reflux you do want the wide bore of a West or Allihn condenser (I also see these called bubble condensers). But if you're just trying to
knock solvent back into a pot then a jacketed coil condenser works pretty darn good.
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
thats exactly right bromic acid.
? graham condensers can't be used for reflux what a load of bullshit
azo
|
|
|
EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
thanks BromicAcid, off-gassing is the opposite of what i want to happen. i will be refluxing a wide variety of different bp compounds but the one
that raises the most concern with me is trying to condense a compound that has a bp of -6C. it's obviously a gas a room temperature but will be
solvated in MeOH, however when the exothermic reaction begins to pick up steam and the MeOH refluxes then there's worry of losing the gas that is
solvated in it. so i'd like to be able to condense both the gas at -6C and the MeOH. so i'm wondering if the friedrich would shine over an allihn in
a situation like that.
of course this also brings up the topic of what coolant to use to feed through the condenser to be able to condense something that has a bp of -6C. i
saw a post on here that had a great listing of different freezing mixtures with the ratios included. NaCl + ice got down to around -20C if i remember
correctly however wouldn't a dewar be needed in that particular example? jon made a good point about using antifreeze + dry ice instead of feeding
that through the condenser, that should reach a cool -30C or so. in fact i remember seeing pics of someone running antifreeze through their condenser
for a distillation here but it was awhile back that i read that thread and can't seem to find that post anymore. the only thing that concerns me with
the use of antifreeze is if it would tear up a plastic fountain pump or any other similar plastic water recirculating pump.
i'm not looking for anything like IPA + dry ice as that is overkill. just wondering which condenser would be better suited for the example above...
and perhaps also if the antifreeze idea would be worth looking into or not
[Edited on 31-7-2010 by EmmisonJ]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
That's not a "load of bullshit", that was the personal opinion of a graduate student who has been doing organic synthesis in an academic setting for
the past five years, and who happens to TA organic chemistry labs ever since.
You should try and express your ideas in a more apropriate manner, this is a science forum after all.
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Amusingly enough if you search Graham condenser on google the first hit is the wiki article on distillation. The reason it pops up is because there
is a picture in the article of someone refluxing toluene using a Graham condenser.
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by EmmisonJ  | i'm also curious, why do so many people slam graham condensers as being nearly useless? from the diagram, they would appear to be the most efficient
at condensing vapors because of the long path the vapors have to travel while being surrounded by the coolant. certainly i'm missing some logic in
this.
[Edited on 30-7-2010 by EmmisonJ] |
EmmisonJ was obviously referring to to the "second configuration" as Wiki calls it (as opposed to the "first configuration" depicted in the picture
you're reffering to) of Graham condenser, in which the vapours to be condensed have to pass through a narrow spiral tube surrounded by a water jacket.
I'd appreciate very much BromicAcid if you can show me a picture on the internet of a setup (made by a professional chemist) which has the "second
configuration" of Graham used for refluxing.
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
BromicAcid and azo, here is an excerpt from "Organikum - Organisch-chemisches Grundpraktikum", 21 Aufl., Wiley-VCH, Weinheim, 2001, pp. 4 -5.
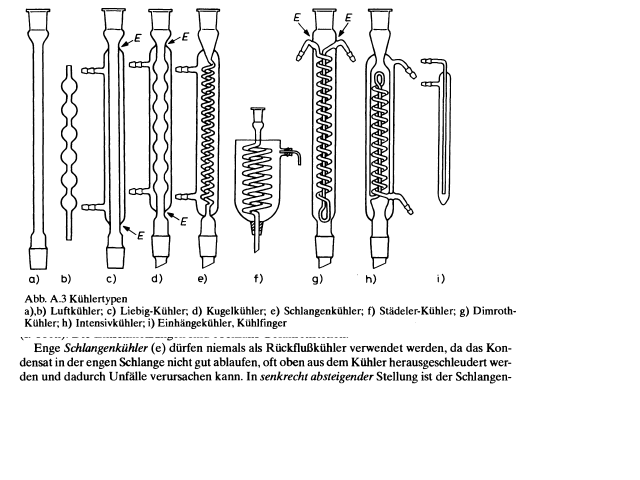
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Gentlemen, it does make a difference whether the coolant is circulated inside the coil or inside the jacket. These coiled condensers come in two
flavors: the one with the coolant in the jacket is not good for reflux, the one with coolant in the coil is good for reflux.
[Edited on 1-8-2010 by entropy51]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
For those who don't speak German, the text says something like
'Narrow Graham condensers (lit. 'snake condensers') may never be used as reflux condensers, as the condensate in the narrow coil cannot run down well,
[and] may often be ejected from the top of the condenser and thereby cause accidents. In vertically descending orientation the Graham condenser is
...'
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Some more info on condensers:
http://www.sciencemadness.org/talk/viewthread.php?tid=2473#p...
|
|
|
BromicAcid
International Hazard
    
Posts: 3247
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Quote: Originally posted by EmmisonJ  | | I'd appreciate very much BromicAcid if you can show me a picture on the internet of a setup (made by a professional chemist) which has the "second
configuration" of Graham used for refluxing. |
I freely admit that I've never heard of this 'second configuration' being called a Graham condenser, I've only heard it referred to at my plant as an
inverted coil condenser and I have never seen someone using this. However, I have to wonder what your qualifications would be for a professional
chemist? There are plenty of idiot chemists that simply get advanced in rank because they have stuck around their companies for so long, believe me,
I know, so perhaps you should have specified a competent chemist? But then what would be the measure for that 
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
Graham is for reflux, it cant be used for fractional destilation. Here is photo of my graham it had both ends the same only size are diferent so it
cant be used for destilation  I read in soviet book "Laboratory work methods"
that graham is for reflux. And i am chemistry student (second year in university) and working in Institute of Solid State Physics (www.cfi.lv) I read in soviet book "Laboratory work methods"
that graham is for reflux. And i am chemistry student (second year in university) and working in Institute of Solid State Physics (www.cfi.lv)
http://img529.imageshack.us/img529/8594/dzesinatajs.jpg
[Edited on 1-8-2010 by Mildronate]
[Edited on 15-4-2011 by madscientist]
|
|
|
EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
nice pic Mildronate, that condenser looks good for reflux but i've heard of condensers that look like that as simply being called a "reflux style
condenser", very generic sounding i know but that's what i've heard them being called.
here is a picture of 2 different graham condensers. the one on the left looks like yours where the coolant goes through the coils and i've heard
called "reflux style condenser", theoretically i would think it would be like a friedrich as far as efficiency goes for refluxing. the one on the
right however is where the vapor itself goes through the coils and the coolant goes through the jacket like an allihn. the one on the right is the
one i hear people talking trash about. i guess i could see how the one on the right could clog easily if a heavy reflux was going because condensate
would be slow to travel down through the thin coils with all those turns and perhaps it would choke easily. also the one on the right would probably
not work at all for distillations? i'm guessing because it'd have to defy gravity to travel upward through the top part of the coils, even if it were
angled i think it'd have a hard time. i'm sure the one on the right would do a great job at condensing because it'd have so much surface area and
the vapors would spent more time in contact with the coolant because of the vapors having to travel through the coils, but that would also be it's
downfall too cuz i think anything beyond a mild mild reflux would choke it, no?

thanks for the link magpie i'm going to read that thread now but so far it's looking like the friedrich is right for me based on BromicAcid's comment
that a wider condenser like an allihn is better for off-gassing, which is the opposite of what i want since i want to condense the solvent vapor but
condense the gas to subzero temps too. i'm going to go read that thread but now am wondering how that "reflux style" graham condenser mentioned above
compares to a friedrich
[Edited on 1-8-2010 by EmmisonJ]
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
I have both these style of condenser(400ml). The one on the right is useless for reflux,but excellent for condensing highly volatile solvents.As a
second stage condenser for ether sythesis for instance.They need to be set vertically or there is remnant in the coil.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I think some of you are mixing up the names here and getting confused.
A COIL condenser is one in which the coolant goes through the spiral and the vapours travel around the sides.
A GRAHAM condenser is one where the vapour travels through the spiral and the water forms a jacket around it. These are also called INLAND REVENUE
condensers.
The Wiki is incorrectly calling both the same thing. If you shop around a lot of the big name glass suppliers, you'll see them making this
distinction. The article has been written by a spazztard with too much time to spend in paint. I'm quite amazed it hasn't been torn apart by some more
knowledgeable chemist as of yet. I have begun instigating that process by flagging the article, adding a note about the inaccuracies and then
discussing it in the talk page. It needs a cathartic purging of it's errors.
If you're refluxing, a GRAHAM condenser (vapours through the spiral) is a bad idea, as there's a high chance of it clogging or flooding. Which will
either blow the liquefied solvent out the top or pop the glass as the pressure builds inside should it clog. You want an Allihn or a COIL (water
through spiral). Allihns in particular are designed for refluxing and a lot of them come with very wide male sockets on the base to fit the equally
wide top of a Soxhlet extractor; which needs to be wide to load the thimble. At the top, you'll notice a lot of them don't have a ground socket, it's
a bare tube. That's because it isn't designed for connection to other glassware, it's intended to go on a Soxhlet for reflux.
I've seen people referring to COIL condensers as Friedrich's before. A genuine Friedrich differs from a coil as the cold element is usually a cold
finger with a spiral groove up the side (not a long tube), and it's a tight fit in the outer glass. They're usually used on rotovaps.
If refluxing is your main deal, get an Allihn. Personally, I'd say get a good COIL condenser and you'll get excellent performance during reflux or
distillation. I can have my plate at 380C boiling sub 100C solvents and they won't get past the first turn or two of the coil.
The jacketed condensers are aimed more towards the major pain in the ass to condense solvents, like ether. My coil will easily do DCM, which boils at
?47C?. COIL condensers are sometimes actually called REFLUX condensers.
This...

is a COIL condenser, NOT a Grahams / Inland Revenue.
The following are the names the manufacturers themselves apply to the glassware, not what I've chosen to call them or worked out from other
references.
QuickFit is an ACTUAL BRAND of glass blown in England, not a generic name for all ground glassware. You can see the brand logo in the following
pictures, it's a big Q with Pyrex QuickFit UK written inside it.
WHAT CHEMGLASS CALL A COIL CONDENSER;

WHAT SIGMA CALL A COIL CONDENSER. THEY ALSO HAVE IN BRACKETS (GRAHAMS), BUT THIS IS A QUICKFIT BRANDED CONDENSER, AND QUICKFIT THEMSELVES DO NOT GIVE
IT THIS NAME, AND HAVE A DIFFERENT NAME FOR THE TWO, AS YOU'LL SEE IN A SECOND;

WHAT SIGMA CALL A GRAHAMS CONDENSER;

A QUICKFIT COIL CONDENSER (I actually have this precise coil, it works beautifully for aggressive reflux & precise distillation. Note the kink in
the end of the spiral, you'll see that on other coils sometimes as a loop. It's a drip tip, there specifically to drip refluxing solvent back into the
centre of a stir vortex or thimble and it keep it away from the tapers, where it'll wash grease back with it; you can see this happening the in the
Wiki image at the top);

A QUICKFIT INLAND REVENUE CONDENSER;

[Edited on 3-8-2010 by peach]
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
My secret santa got me one of these;

and it works really well as a reflux condenser.
Not tried yet but I'm sure that it would also make a good product condenser.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
BaFuxa
Hazard to Self
 
Posts: 61
Registered: 18-9-2017
Location: Mars
Member Is Offline
Mood: Buzzing
|
|
I own a friedrichs condenser. I think it offers more versatility. It is a robust condenser that you can use for both distillations and refluxes.
Doug's lab used a friedrichs to distill diethyl ether. It is also more sturdy and thicker than an allihn, thus you can use it for vacuum
distillation/refluxes without having to worry about it. It also offers more surface area, length for length, than an allhin. An allihn is just
slightly improved liebig and it is not really meant for distillation. Lastly a friedrichs is fairly cheap on aliexpress. Mine is a synthware and I am
absolutely happy with it. Beautiful piece of glassware.
Potential counts for nothing until realized.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Sulaiman,
Alas, despite the label, this is not a Graham Condenser. It a appears to be a Dimroth type.
Both more expensive, and more versatile, than an actual Graham.
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by zed  | Sulaiman,
Alas, despite the label, this is not a Graham Condenser. It a appears to be a Dimroth type.
Both more expensive, and more versatile, than an actual Graham. |
Yes it is Dimroth-like ... get one quick before the seller realises 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|