lol
Harmless

Posts: 16
Registered: 22-7-2010
Location: i'm from holland
Member Is Offline
Mood: No Mood
|
|
wheres this error?
hi folks,
im new to this and i could use some help....im trying to have the numbers add up....
now im trying to make this recipe: PTC Methylenation of Catechol (Using CTAB) (http://www.erowid.org/archive/rhodium/chemistry/methylenatio...)
it reads; and 3.2 grams (0.1 mol) of cetyltrimethylammonium bromide (Cetrimonium Bromide, CTAB)
is this a typo in the mol part? all the sources tell me that the CTAB is 364,45 grams per mole
[Edited on 25-7-2010 by lol]
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Your wanting to make MDMA aren't you?
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The Rhodium Archive isn't known for its fastidious accuracy.
It is generously provided free of charge, and it contains lots of interesting information,
but a fair percentage of the material presented contains errors. Or, alternately, has no real practical applications.
Try to access an original paper on the subject.
Peach has a recent series of posts on the subject you are trying to grok. Maybe he knows something.
Methyl aryl ether demethylation of 4-Allyl-2-methoxyphenol - a LONG discussion
[Edited on 25-7-2010 by zed]
|
|
|
lol
Harmless

Posts: 16
Registered: 22-7-2010
Location: i'm from holland
Member Is Offline
Mood: No Mood
|
|
thanx, enough to read there 
@mewrox99 i'll make anything as long as its edible
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Uh oh.
Great start. Welcome to SM.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
lol
Harmless

Posts: 16
Registered: 22-7-2010
Location: i'm from holland
Member Is Offline
Mood: No Mood
|
|
thanx 
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
-.-'
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by lol  | hi folks,
im new to this and i could use some help....im trying to have the numbers add up....
now im trying to make this recipe: PTC Methylenation of Catechol (Using CTAB) (http://www.erowid.org/archive/rhodium/chemistry/methylenatio...)
it reads; and 3.2 grams (0.1 mol) of cetyltrimethylammonium bromide (Cetrimonium Bromide, CTAB)
is this a typo in the mol part? all the sources tell me that the CTAB is 364,45 grams per mole |
I had a look at the original literature and it indeed says "3.2 grams (0.1 mol)" so the error is in there. I believe they mean 3.6 g (0.01 mol) as in
the next example, after all it is a catalyst. OTOH this is about emulsions so you never know. Furthermore, maybe I'm blind, but I didn't see any
alkylation of diphenols in that paper, only of phenol. So I wonder where the 90% yield comes from? It would be great if you try this method and report
success or failure. It might be of advantage to start at 1/5th scale or something.
@mewrox99: Please stop that shit. You don't have to prove that you know what can be done with benzodioxole. It's none of your business and you're
probably wrong to boot. Could as well be MDA, mollinedine or whatever. This is interesting chemistry, the question of the OP was perfectly valid and
he found an error in a published procedure. AFAIK that's more than you did. If you feel the need to police the board apply for moderatorship or use
the "Report this post" button. Stop cluttering the board with non-chemistry drivel.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
You could try the DMSO / DCM method first if you don't have PTCs to hand.
He may have bought some plain Jane catechol and be attempting to close it to 1,2-methylenedioxybenzene / 1,3-benzodioxole to see if those ideas even
work. Which I say is fair game, I'm sick to death of seeing that kind of stuff copypasta'd all over the web as fact when no one has actually bothered
trying it.
It will be a mistake and they'll mean 0.01 moles.
0.1 moles would be a lot.
I've not done much with methylenation myself, but I suspect even 0.01 moles is a decent amount. PTCs tend to be used in very tiny quantities.
This is part of the reason for me running that demethylation. I see people constantly going on and on about it, yet it also seems that no one has
actually tried most of the ideas regarding hard lewis acid demethylation of this kind of thing.
That particular substrate is interesting as journal entries routinely use substrates that only feature one functional group, making them far easier to
take a shotgun, aggressive approach to them. The same methods used on the different substrate don't seem to work so well. It makes the chemistry a
little more interesting, thinking about the details of how something will be attacked as opposed to the mechanism alone.
If anyone happens to have some TBAI to hand, shoot me a PM. I have a container full of TBAB, but the guys I buy it from only sell the iodide in huge
drums.
I plan to dump a lot of far briefer and higher quality work on that thread. But I'm currently waiting for money to come through from selling some
welding gear, need my glass repairing and I have micro-scale short path glass to come as well. The woman I bought it from has been on holiday the last
two weeks, is now uber busy packing boxes, so I have to wait until the end of the week before she'll even reply, and then I have to wait for that box
to get packed and sent. So I'm having to patiently wait.
I want to run that demethylation to the letter and see that specific catechol in a high purity, unoxidized form, so I can finally say "Does work" or
"Doesn't work".
Got the 99.998% N2 atmosphere, cyclohexane, pharmacopedia grade substrate, sublimated iodine, atomized aluminium and TBAB all on the shelf. I can
vacuum distill, collect different fractions via a multiway receiver and have flasks down to 5ml. But I'd like to knock out the TBAB variable by
replacing it with TBAI, so it's letter perfect and there can be no more "Oooo but...."
I'd like to try making Dopamine with it, so I can seal it in a tiny ampoule and start a collection of "Things that make me think"

Eugenol;
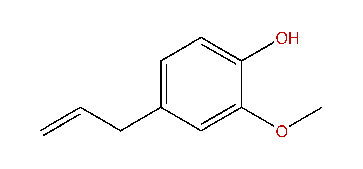
NEUROSCIENCE TRESHAR OFF THAR PORT BOW! HOIST THAR MAIN SAIL AND BE LOADIN' THE IRON WITH GRAPE!

[Edited on 26-7-2010 by peach]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | | Furthermore, maybe I'm blind, but I didn't see any alkylation of diphenols in that paper, only of phenol. So I wonder where the 90% yield comes from?
It would be great if you try this method and report success or failure. It might be of advantage to start at 1/5th scale or something.
|
That this procedure is not about methylenation of catecholes was already settled previously. It is about alkylation of phenols and I can't see how it could be applied to methylenation of catechols which at the
reaction conditions would be doubly deprotonated hence could not be carried in the organic phase with the quaternary ammonium counteranion, but would
rather stay in the aqueous phase (hence not get into the contact with the alkylating reagent). How that mistake come into the Rhodium archive is easy
to understands as there are many such. The original poster was obviously too lazy to either check the reference or to UTFSE. Not that I would expect
anything better from someone who believes procedures are recipes.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
*sigh*
Thanks for the explanation, makes sense. IMHO there are errors like wrong speculation and there is fraud like this totally made up procedure
including fantasy yields. Or was the reporter confused by the 2Ph-OH + Cl-CH2-CH2-Cl -> Ph-O-CH2-CH2-O-Ph reaction? I wish the Erowid guys would
wikify the Rhodium archive.
As for the wrong lingo and the inability to look up the original procedure - what do you expect from a newbie with no formal training at all? I guess
that's what the "Beginning" subforum is here for: To learn these things.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Avast me hearties, this thar file be hosted here on our own good ship, the lady Madness... a fine vessel she be... arr..... and yar be a naughty boy
if yar be following this file to the lettar, and likely in receipt of the cat'o'nine
Nicodarm, could the reaction not be occurin' at thar phase boundary? I expect it be enargetically unfavarable given the charge difference. And that
yar be right about the blanket PTC approach to phase differences. But be barin' in mind thar that the diphenol catechol be likely soluble in polar
aprotics, such as ethyl acetate and thar halogenated methyls. Thar be a reference from one of these articles -----> hear. But I not be possessin' thar Athens login or 'hack' route to thar journals, and so cannot be determinin' if it be a diphenol or not.
Once me glasswarez be arrivin' from the new world, I be able to acquire a pyar sample o' the catechol in question to assay these thar solubility
propteries, but I be testing it roughly thus far and strongly suspectin' be it polar aprotic soluble. Me current sample be over oxidized. Me clamp
stand be on lone as well, so I can not be runnin' the demethylation for a purer sample I can distill without me expensive glass topplin' in thar drag
and foam.
I be interested in hearin' from any old salts who be possessin' superior analytical equipment to be sure o' me product, e.g. optical spectroscopy,
mass spec ar NMR. PM me and I be sending yar a sample to run thru when it be done. I be fairly sure already, as it be smellin' correct, behavin'
correctly at thar specified temperatuars, oxidisin' and respondin' to light or thar atmosphere and performin' in a ferric chloride phenol test, but
mar evidence never be barnacles on me hull. Arrr....
I also be aftar some TLC plates, but thar box price be beyond me already stretched budget, I be spendin' a fortune in gold pieces of glassware, pumps
and cylinders recently, thousands. If anyone be havin' unused plates or wantin' to split a box, that thar be interestin'.
A life on the ocean wave! A home on the rolling deep! Where the scattered waters rave, and the winds their revels keep!

[Edited on 26-7-2010 by peach]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Check Ebay. TLC plates can be had for ~$10. Unreacted phenol is removed by base washes, oligomers will not distill. Single spot TLC and the correct
boiling point should be proof enough.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by turd  | | Check Ebay. TLC plates can be had for ~$10. Unreacted phenol is removed by base washes, oligomers will not distill. Single spot TLC and the correct
boiling point should be proof enough. |
I just did a search for TLC under Healthcare, Lab & Life Science. The cheapest is $23.90, and it's not TLC plates. And I don't live in the US, so
a number of the sellers won't ship to me.
[Edited on 26-7-2010 by peach]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
One good tip for Ebay: Patience.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  | | Nicodarm, could the reaction not be occurin' at thar phase boundary? I expect it be enargetically unfavarable given the charge difference. And that
yar be right about the blanket PTC approach to phase differences. But be barin' in mind thar that the diphenol catechol be likely soluble in polar
aprotics, such as ethyl acetate and thar halogenated methyls. Thar be a reference from one of these articles -----> hear. But I not be possessin' thar Athens login or 'hack' route to thar journals, and so cannot be determinin' if it be a diphenol or not.
|
Good point and interesting find that paper (BTW, you can use the References section for retrieving papers). I still think that the dianion can not be
carried over into the nonpolar phase efficiently enough for the nucleophilic substitution to occur in there. I never saw claims of comparatively polar
dianions being carried over under PTC conditions (and I did read some review papers on the topic). Of course it would be possible that some of the
monodeprotonated catechol resulting from the (de)protonation equilibrium is caried over and reacts, but in 20% NaOH(aq) this equilibrium is close to
negligible. And here might lie one possible explanation for the Tet. Lett. results. The difference in the reaction conditions for the Tet. Lett.
methylenation is in that here an aqueous solution of the disodium salt of catechole is formed without much NaOH excess (only 25% excess). This is
unlike in the PTC alkylation discussed earlier where a huge excess of NaOH is used (a 25-fold excess!). With such an excess, most of the alkylating
reagents that would dissolve in the aqueous phase would react with the hydroxide instead of the phenoxide. On the other hand, in the Tet. Lett.
methylenation, any CH2Br2 that dissolves in the aqueous phase (and its solubility in water is significant: 1.1 g/100g at 25°C) can only react with
the phenoxide nucleophiles, thus giving the benzodioxoles. If this is the case then the PTC has no role (except for increasing the surface contact via
emulsification). In fact, in my experience I found the PTC catalyst is not even needed in an allylation of a phenol I once had to do. If I used a
stoichiometric amount of NaOH(aq) and stirred the so formed aq. phenoxide solution with allyl bromide, I got the same result with or without adding
TBAB. I later observed the same with alkylating aq. sodium phenoxide with 1,2-dibromoethane - PTC was not needed and I concluded that the alkylation
thus occurs in the aqueous phase. In fact, if this is true then catechols can be efficiently methylenated with CH2Br2 already in methanol with KOH as
a base. Unfortunately, that paper, like most letter articles, lacks some scientific strictness and gives no results for the control experiment (one
without PTC catalyst added).
The other possibility is the one mentioned above. Where there is no large excess of the hydroxide there is always some monodeprotonated catechole
present and this can be carried over via PTC just like any other normal phenoxide is.
Also, it is important to note that CH2Br2 methylenates catechols way more efficiently than CH2Cl2, and that this paper gives no indication that using
CH2Cl2 under the same conditions would give anything useful.
Attachment: 3489-3490.pdf (110kB)
This file has been downloaded 545 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
This is something I discussed on other forums, but received absolutely no answer to, the amount of base required and the solvent.
CH2Cl2 is likely to yield far more poorly than the other halides.
I have no practical experience with diphenols and their methylenation at present, so I don't really want to spend too long discussing things like the
PTC, as I find it tends to yield the philosophical equivalent of intractable tar; rubbish.
If I ever have any catechol to hand that I wish to bridge, I'll give it a try. If anyone has some straight catechol, I could give it a go with that.
What's that mention about the PTC poisoning by iodine?
|
|
|