Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Reactions with sodium benzoate
Does anyone know some reactions I can do with sodium benzoate?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yea hundreds...
Note my answer is about as useful as your question. Chemistry is not a subject to be vague with.
[Edited on 28-6-2010 by DJF90]
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Can you list some of them that can be done with easily accessible chemicals?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Start off by isolating the acid itself. Check the thread on benzoic acid => benzamide => aniline an initial idea.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Quote: Originally posted by DJF90  | | Start off by isolating the acid itself. Check the thread on benzoic acid => benzamide => aniline an initial idea. |
Thanks for the tip 
|
|
|
querjek
Hazard to Self
 
Posts: 76
Registered: 26-8-2008
Member Is Offline
Mood: No Mood
|
|
Form an ester such as ethyl benzoate from EtOH and H2SO4.
Degrade it into benzene with Na/KOH or CaO.
it's all about chemistry.
|
|
|
Taoiseach
Hazard to Others
  
Posts: 241
Registered: 16-3-2008
Member Is Offline
Mood: No Mood
|
|
Use it to make benzene, its a pretty straight forward synthesis and benzene ist about the most valuable chemical you can obtain from it.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Besides the already mentioned uses in the preparation of benzoic acid and benzene here are a few more ideas:
a.) Alkylations with reactive alkyl (pseudo)halides. Carboxylate salts become moderately good nucleophiles in aprotic polar solvents and they can be
alkylated to form the corresponding esters, for example benzyl benzoate from benzyl chloride.
b.) Preparation of carboxylate based metal complexes (the benzoate anion can be used as a ligand for some metals). Similarly, benzoate salts can be
made using methathesis reactions.
c.) Preparation of benzoyl chloride or benzoic anhydride.
d.) Under some conditions alkylmagnesium halides react with metal carboxylates to give the corresponding ketones, acetophenones in this case. The
problem is usually the low solubility of the sodium salts in the ether solvents and the low reactivity. Alkyl lithiums react much more rapidly to give
the same ketones.
f.) Sodium benzoate can be "activated" by certain reagent to form mixed anhydrides to be used in one-pot syntheses of amides, esters or other carbonyl
compounds. Reagents that can be used include (m)ethyl chlorofomate, pivaloyl chloride and possibly other such.
g.) Electrolysis experiments (see Woelen's posts about it).
h.) Research of bromobenzene synthesis via the Hunsdiecker reaction. Sodium carboxylates react slowly in this reaction if they do at all, certainly
much more slowly than the usually used silver salts. Already silver benzoates are troublesome in this reaction so this would be something not yet
described and interesting to research.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
|
Thread Moved
29-6-2010 at 00:22 |
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The stuff is used as a soluble food preservative, particularly for sauces. I think I have also seen it used as a temporary preservative for biological
specimens used in biology teaching laboratories.
After degrading the stuff to C6H6 as above, you could then nitrate it with HNO3 and a small amount of H2SO4, to get C6H5NO2. This can be used as a
qualitative analytical reagent, or reduced to aniline, C6H5NH2. From that, you could get azo compounds. Or you could sulfonate it with a larger
quantity of H2SO4 to get benzenesulfonic acid, C6H5-O-SO3H.
Benzoates can themselves be nitrated to m-nitrobenzoic acid. Also, using LiAlH4, or on an industrial scale catalytically or with nascent H2, they can
be reduced to benzyl alcohol; or, using Li[AlH(OC(CH3)3)3], reduced only to benzaledyde, C6H5CHO, which is a very important intermediate product,
including for certain drugs.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
How could I make benzyl chloride from benzoic anhydride? Is benzoic anhydride just dried benzoic acid?
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
No benzoic anhydride is not simply dry benzoic acid, it is the dehydrated form of benzoic acid (theres a O where the OH's would normaly be). As for
benzoyl chloride, acyl chlorides can be made from the reaction of dry HCl with the acid anhydride, but this would be tricky since benzoic anhydride is
a solid.
The difference,
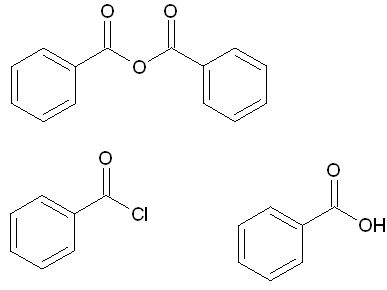
Edit: Whoopsidoodle, thanks for pointing that out I simply assumed he was referring to benzoyl chloride since he was asking about benzoic anhydride.
[Edited on 12-10-2010 by mnick12]
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
Noting that benzyl chloride and benzoyl chloride are not the same thing.
|
|
|
Random
International Hazard
    
Posts: 1120
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
I made a mistake, it was benzyl chloride actually 
Could I make vanillin or vanillic acid from it as they have similar structures?
|
|
|
spirocycle
Hazard to Others
  
Posts: 197
Registered: 29-9-2010
Member Is Offline
Mood: No Mood
|
|
you could, but theyre not that close in stucture so it would be pretty intensive
youd probably be better off starting with benzaldehyde, phenol, or anisole
|
|
|
MadHatter
International Hazard
    
Posts: 1339
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Whistle mix !
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|