MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Help With Organic Nomenclature
I am trying to help someone with their organic chemistry. I am only in my first semester of organic (and it is the beginning at that). They need the
structure of a compound:
3-(2-methylcyclobutyl)-4,6-diethyl-7-(l, I-dimethylethyl)decane
I don't know what the "capitol i" means, though. Also, how would I make this the levo- isomer?
I have this so far: *Damn, I messed up. Shift all substituents up one carbon*
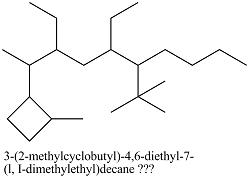
Thanks in advance.
[Edited on 1-28-2010 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
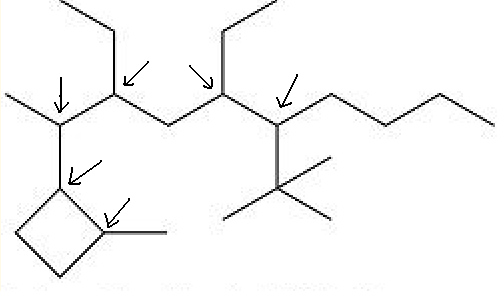
I would call the compound drawn 2-(2-methylcyclobutyl)-3,5-diethyl-6-t-butyldecane and expect to be perfectly understood, even if it is not the latest
fashion in nomenclature.
So try drawing again. 1,1-dimethylethyl is tha same as t-butyl, but looks uglier, to me.
[Edited on 28-1-2010 by Paddywhacker]
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
The name of your compound can vary between some names, all will depend on the convention that you use. For exemple, at my school we are oblige to use
the IUPAC name convention.
This is the step to name a molecule by this convention.
1. Find the longest chain of carbone.
2. Find the side to start giving the number of the ramification.
(ex: Smaller number for OH group, for the double and triple liasion or try the have the smallest number in total) ( they have also other things, but
you can start with this)
3.Right the molecule name in alphabetical order for the ramification and not in the order that they come on the molecule.
(exception: For the alphabetical order, ''di'' and ''tri'' don't play a role, but we have to consider ''cyclo'' and ''tert-''. I don't know why, but
someone have decide this...)
The bad thing is that I never name a ramification with a ramification (2-methylcyclobutyl). But let say that it really have to be place in
alphabetical order, it will give.
3,5-diethyl-2-(2-methylcyclobutyl)-6-tert-butyldecane
The thing that I'm not sure is that complexe ramification go always on the begining of the name...
Did you seem the possible isomer???
isomer : Cis)trans or E)Z
optical isomer: R)S or l)d
Because your drawn have the posibility to be trans or cis (2-methylcyclobutyl), but because the drawn is not in three dimentions, you can't tell this.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That compound appears to have 6 optically active (stereogenic) carbons. Have any of the 64 possible stereoisomers been actually synthesized and
characterized?
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
It's starting to look like one of those theoretical molecules that some folk make because they want to call it penisane as it looks like a penis.
EDIT: I am the champion of run-on sentences.
[Edited on 28-1-2010 by psychokinetic]
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
As JohnWW said, they have 6 carbon with 4 different ramification. We call this chirality or ''asymmetric carbon atom''.
The probleme is that you can't tell the final form of the molecule because it is in two dimentions, so you can't tell more on this compound.
On the other drawn, know you can tell the optical isomer (maybe you will only see this at the end of the semester...)
(S)-1-bromopropan-1-ol
 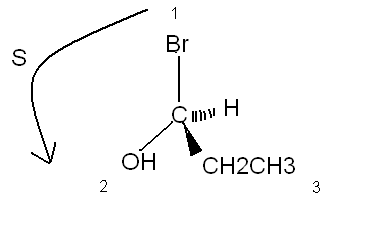
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I think you guys may not fully understand what I am saying. I was given the name of the compound and I needed to draw the structure. That picture
was my primary attempt. And I don't know what the capitol i means in the nomenclature. Was that a typo and the L and i were meant as 1s? Maybe
that's why it confused me.
Thanks for all the help. I really appreciate it.
P.S. In short, the information given to me was the name of the compound and I was trying to come up with the bond-line structure.
[Edited on 1-29-2010 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
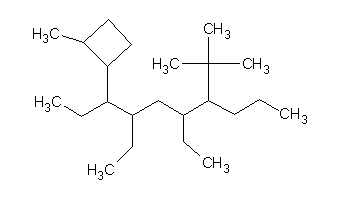
Sorry, I read your post too fast.
I talk with my teacher and the ''I'' in the name is a mistake. I am pready sure that it should be the number one. She also told me that the ''complexe
ramification'' always go at the beginning of the molecule.
Your other question was how can this molecule be levo- isomere?
The bad thing is that l/d is not really in relation with R/S so even if you drawn the molecule in 3 dimention, you can't say if is levo or dextro. The
only way to know is by making the light deviation test with differente isomer to see the light deviation. Levo = left deviation of the light.
I espect that it help.
[Edited on 30-1-2010 by Bikemaster]
[Edited on 30-1-2010 by Bikemaster]
|
|
|
VestriDeus
Harmless

Posts: 16
Registered: 15-11-2009
Member Is Offline
Mood: No Mood
|
|
I got:
3,5-diethyl-2-(2-methylcyclobutyl)-6-tert-butyldecane
Your teacher's a [insert derogatory name here]
And if he/she doesnt take the "tert" part, then he/she should get out of teaching.
|
|
|