wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Removing alkanes from an amine
How can I replace the two alkans with hydrogen on the amine (the right hand N) in this azo dye?
I searched but found no method, I guess its probably difficult or requires exotic reagents. I do not want to change any other groups in the dye.
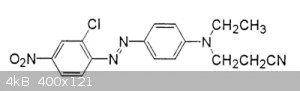
Sorry I don't why the system keeps putting the extra images in the post
[Edited on 4-1-2019 by wg48temp9]
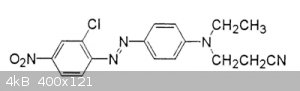 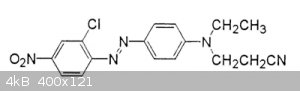
[Edited on 4-1-2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
N, demethylation removes methyl groups but I don't know if it extends to other alkyl groups like deethylation or depropylation but it might be a good
place to start.the only things I've heard of people wantinh to N,demethylate is opiates.O, demethylation is much more common.anyway polonovski
demethylation,opiate chemistry and N, demethylation are three places I would suggest that you have a good look at.i think it involves oxidizing the
amine with h2o2 to form an N,oxide and ferrous sulphate to remove the alkyl. I always wondered if this also works on ethyl and propyl groups.good
luck.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
That cyanoethyl group looks like it can be cleaved back to acrylonitrile, as is the case when it is used as a protecting group on phosphates. (I will
look up some conditions for cyanoethyl anilines tomorrow).
And the stereotypical reaction to demethylate amines is the von braun reaction, or it's more modern modification using some alkyl chloroformate
(methyl, ethyl or isopropyl).
https://en.m.wikipedia.org/wiki/Von_Braun_reaction
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Does the Von braun alkyl cloroformate rxn extend to ethyl,propyl,butyl groups?
[Edited on 5-1-2019 by draculic acid69]
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Thanks for the suggestions guys.
i looked at the N-oxide method again. I assume as my dye is not water soluble I need an organic peroxide but I suspect almost any organic peroxide
will do. Then I remembered OPs are available OTC as catalysts for resins. They are expensive and are usually diluted (not in water) but just
affordable for me as I only need a few tens of grams.
I also want to try hydrolyzing the nitrile.
Ok its probably less than 50% MEKP: 130ml £2.50 at https://www.mbfg.co.uk/butanox-m50-130ml.html?gclid=EAIaIQob...
[Edited on 5-1-2019 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Find another starting material.
Probably easier to build your target material from scratch, than it is to modify that tertiary amine.
|
|
|