Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
disodium phosphate
I recently have made some Na2HPO4 using 11M H3PO4 and NaOH as a precursor to microcosmic salt. My method was to make up about a 30% NaOH solution
then slowly add it to the phosphoric acid with good agitation. Periodically I checked the pH with litmus paper. Theoretical endpoint was about 9.3
pH, but I could only approach this roughly using my crude method. After filtering the mother liquor, the crystals formed immediately upon cooling and
have been drying slowly at room temperature for about a week. My humidity meter has been reading 28-29% RH. Below is a picture of the crystals so
far. They seem to be dehydrated on top but still carrying a lot of water below the surface.
Any comments? Any suggestions for a better way to make this compound?
[Edited on 27-12-2008 by Magpie]

|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
What a coincidence! Right behind me, on the room heater, is standing a bowl of a very similar compound- I have made sodium dihydrogen phosphate, from
H3PO4 and NaOH.
85% H3PO4 was weighed carefully, as well as solid NaOH (0,5 mol each).
Both were diluted with about two times their own weight of water, but the solutions still boiled upon careful mixing.
I did not check the pH of the solution, but tasted a little bit of it- it tastes both salty and a bit sour.
(You see, I have confidence in my stochiometric calculations ) )
The solution is slowly crystallizing. The solution has a strong tendency to form a crystal skin on its surface that hinders further evaporation of
water. I often stir it to break the crystal skin.
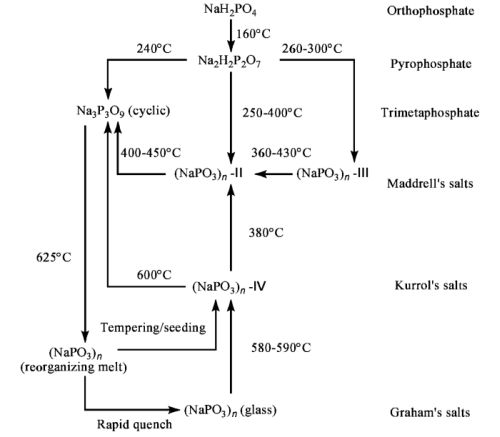
I intend on calcining this to Na3P3O9, cyclic sodium trimetaphosphate, the compound I intend on using as the precursor to phosphorus.
Many types of polyphosphates with different properties can be obtained from monosodium phosphate by heating and cooling slowly or quenching a melt.
I have a diagram of this somewhere.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Diagram attached.
The cyclic Na3P3O9 is obtained by slowly heating monosodium phosphate to 550°C, and letting it cool down slowly again, in the furnace.
The long-chain polyphosphates, on the other hand, are made by quenching a NaPO3 melt.
Magpie, your disodium phosphate looks very similar to my monosodium phosphate. The tendency to enclose moisture under a crystal skin is also there.
Why did you make disodium phosphate? Microcosmic salt is NaNH4HPO4, right? You'd need monosodium phosphate to attach the ammonium in the form of
ammonia.
Except you make (NH4)2HPO4 next and mix it with your disodium phosphate. Is that what you want to do?
[Edited on 28-12-2008 by garage chemist]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, this is quite a coincidence, garage chemist! My lab is a little cold right now so I'm entertaining myself by growing some crystals. I'm going
to break this crystal matte up good in an attempt to get it to dry thoroughly.
According to Dr Ure, in an 1827 text, available on Google books, "...microcosmic salt is best made by mixing equal parts of phosphate of soda and
phosphate of ammonia in solution, then crystalizing. A faint excess of ammonia is useful in the solution."
In the same book Berzelius instructs to make it out of sal-ammoniac and phosphate of soda.
I have elected to make it according to Dr Ure. Diammonium phosphate is cheaply available at my local beer/wine brewing supplies shop where it is sold
as a nutrient.
I'm going to use the microcosmic salt for blowpipe testing on some ores where it serves as a flux. It is also historically interesting as a precursor
for phosphorus used by the alchemists.
Is there a particular reason why you chose Na3P3O9 for your precursor? Why not just use sodium hexametaphosphate, (NaPO3)x, ie Calgon?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Calgon, actual calgon, is not available OTC here.
There are products with the trade name "calgon" on them, but they are free of phosphates due to environmental regulations (phosphates cause algal
blooms in rivers).
It sounds strange, but there's no OTC source of a pure phosphate here. Fertilizers are full of impurities since they are made from wet process
phosphoric acid.
Sodum hexametaphosphate is a type of glassy soluble polyphosphate similar to the sodium trimetaphosphate I am making (also derived from MSP). Since
STMP is crystalline instead of glassy, I think it will be easier to powder. It has to be intimately mixed with kieselgur and aluminium, obviously.
STMP and Calgon will give the same product on heating above 650°C anyway, the glassy NaPO3 melt.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Very useful diagram garage chemist!! Thank you for posting that.
I have about 5lbs of monosodium phosphate I purchased off a member here a year or so ago, what to do with it, what to do with it 
So, I am to assume that you will be doing the white phosphorus preparation soon?
I would've liked to do it, but I am currently entertained with the preparation of a different allotrope 
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I want to develop two methods of P4 production, a simple, small-scale one using an oxy-propane welding torch as the heat source and a thin quartz tube
as the vessel, and a larger-scale one using the tube furnace.
My biggest problem is: what am I going to use as the vessels in the future.
I'll use quartz glass now, which will only hold up to a few preparations due to the Na2SiO3 byproduct fusing itself to the walls and causing
devitrification of the quartz glass.
I wish I could make retorts out of a refractory, thermal shock-resistant ceramic material, this would hold up so much better than quartz.
If the qartz gets really badly attacked (my test tube is already frosted on the inside anyway due to an experiment on potassium preparation) I'll
eventually buy some raku clay (should be somewhat resistant to thermal shock in fired state) from a pottery store, make a plaster mold for slip
casting, build an electric kiln and start making my own chemlab pottery. All for phosphorus.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Garage chemist have you considered the use of alumina combustion boats as made by Saint Gobain. VWR claims they are good for use up to >2000C?
I have also thought about making my own clay retorts using castable fireclay. Have you considered it? I have no experience in making ceramics, so
this is a skill I would first have to master. As you say, all for making one product.
[Edited on 27-12-2008 by Magpie]
[Edited on 27-12-2008 by Magpie]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I have no source of alumina boats, and even if I had, the mix is likely to spatter and ruin the quartz tube anyway.
Also, glass (the Na2SiO3) fumes (evaporates) noticeably above 1000°C.
I thought of adding either so much kieselgur that the glass (before the reaction: NaPO3 glass, after the reaction: Na2SiO3 glass) will be held in the
pores like in a sponge and not touch the walls too much.
Or I could add an inert diluent like Al2O3 to do the same job.
There is also the issue of removing the slag after the reaction.
A solid lump of slag would be almost impossible to remove. A porous plug consisting mostly of kieselgur would be much more convenient.
[Edited on 28-12-2008 by garage chemist]
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
| Quote: | Originally posted by Magpie
I recently have made some Na2HPO4 using 11M H3PO4 and NaOH as a precursor to microcosmic salt. My method was to make up about a 30% NaOH solution
then slowly add it to the phosphoric acid with good agitation. Periodically I checked the pH with litmus paper. Theoretical endpoint was about 9.3
pH, but I could only approach this roughly using my crude method. After filtering the mother liquor, the crystals formed immediately upon cooling and
have been drying slowly at room temperature for about a week. My humidity meter has been reading 28-29% RH. Below is a picture of the crystals so
far. They seem to be dehydrated on top but still carrying a lot of water below the surface.
Any comments? Any suggestions for a better way to make this compound?
[Edited on 27-12-2008 by Magpie] |
Personally, I would save precious NaOH for when you really need it. Like making soothing lye soap with shea butter for your shower pleasure Seriously, Sodium carbonate is cheaper and does the job here. I don´t have my
acidic constants available but I beleive the first two hydrogens of orthophosphoric acid are more acidic than carbonic acid while the third one is
not. Simply adding strong sodium carbonate solution to diluted phosphoric acid until the fizzing stops should suffice. I also believe that dibasic
sodium phosphate has wild solubility changes due to temperature. So boiling the neutralized solution toward saturation then cooling to near 0 Celcius
should deposit most of your dibasic phosphate as crystals. Of coarse they will be hydrated. Seriously, Sodium carbonate is cheaper and does the job here. I don´t have my
acidic constants available but I beleive the first two hydrogens of orthophosphoric acid are more acidic than carbonic acid while the third one is
not. Simply adding strong sodium carbonate solution to diluted phosphoric acid until the fizzing stops should suffice. I also believe that dibasic
sodium phosphate has wild solubility changes due to temperature. So boiling the neutralized solution toward saturation then cooling to near 0 Celcius
should deposit most of your dibasic phosphate as crystals. Of coarse they will be hydrated.
Fellow molecular manipulator
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
| Quote: |
Personally, I would save precious NaOH for when you really need it. Like making soothing lye soap with shea butter for your shower pleasure Seriously,
Sodium carbonate is cheaper and does the job here. I don´t have my acidic constants available but I beleive the first two hydrogens of
orthophosphoric acid are more acidic than carbonic acid while the third one is not. Simply adding strong sodium carbonate solution to diluted
phosphoric acid until the fizzing stops should suffice. I also believe that dibasic sodium phosphate has wild solubility changes due to temperature.
So boiling the neutralized solution toward saturation then cooling to near 0 Celcius should deposit most of your dibasic phosphate as crystals. Of
coarse they will be hydrated.
|
I never worry about using NaOH as it is cheaply available to me at my hardware store. But the economics of your method are surely favorable. I do
like the cessation of fizzing as endpoint marker. Sounds good.
As I neared the endpoint of my neutralization of the warm solution it began to cloud up, appearing oversaturated. But rather than dilute it I just
heated it to near boiling and filtered it into the crystalizing dish. The filtrate was clear and crystals formed right away upon cooling. After I
broke them up yesterday they are dehydrating quite nicely to a white powder.
|
|
|