| Pages:
1
2 |
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Your right, P487 is nice work!
Normally I will try to avoid threads like this, but I am curious (mechanism-wise) about the heat + hv. I have seen product distributions like this
with truxillates/truxinates (compromising between thermal and photochemical routes). It would appear that careful optimization of temperature while
the radiative wavelength and flux are fixed (or vice-versa) might give a tuned reaction yielding only two products, one of which in excess?
Immobilizing one of the reactants on a solid phase comes to mind. The concept might be applicable to more interesting (and legal) targets.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
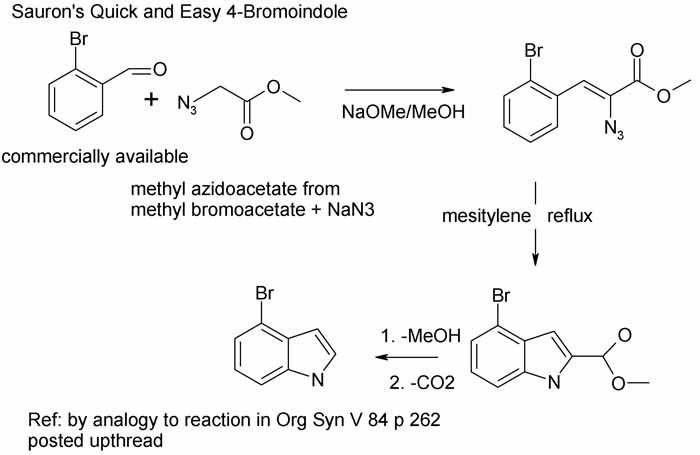
Hemetsberger-Knittel Anyone?
Here is reaction scheme for the easiest indole prep I have ever seen.
If one simply must kvetch, the bromoacetates are nasty lachrymators. But other than that I see little wrong with this short sweet prep.
It is clearly related to the prep employed in the 1994 SynLett paper posted above.
[Edited on 26-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
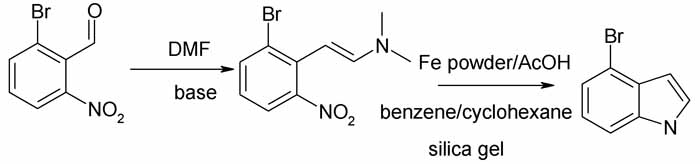
Another look at that precursor in the 1994 SynLett paper (see upthread)
Might not this be the product of base catalyzed condensation of a benzaldehyde with DMF?
If so then the real starting material is 2-bromo-6-nitrobenzaldehyde.
And this one rather than the Hemetsberger-Knittel abovethread, takes pride of place.
In both cases the reaction is between benzaldehydes and a carboxylic acid derivative. On the one hand an ester of a substituted acetic acid, on the
other hand, a formamide (formic acid amide).
NOTE: My speculation was close but not quite perfect.
The ref.7 in SynLett 1994 eventually led back to three papers in a series in Chemical & Pharmaceutical Bulletin, open access English language in
Japan.
The substrate is a substituted nitrotoluene and the reagent, DMF-DMA, dimethylformamide dimethyl acetal. This reacts in DMF as solvent to give 90% of
the enamine and the enamine is easily cyclized to 4-substituted indole. See the scheme and three posted papers downthread.
[Edited on 27-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
If I was going to kvetch, it would be about the N3 group rather than the bromo-acetate. The words This reaction should be performed behind a
safety shield are more worrisome than need a fumehood. Useful when dealing with the pyridine instead of benzene ring, as the
heterocycle is more difficult to deal with than the benzene, with many of the normal routes to indoles not working well. The nitro-benzaldehyde/DMF
would be easier if one were after the vanilla indoles.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Well, I saw that warning, but evaluated it as pro forma, given that no explosions were reported. the azidoacrylates are unstable, and tend to evolve
N2 slowly while you watch. In one example with a different substrate, the reaction mix frothed out of the pot. None of that seems very nerve wracking
to me.
When I was a RA in the 70s there was a postdoc in the same group who was making a series of small ring N-heterocycles, and used a lot of NaN3. His
products often detonated (or deflagrated) in the drying oven. So I have been around azides, and am not easily intimidated. I have also been around
long enough to know when Org Syn's editors are crying wolf for fear of liability lawsuits.
All that being said, now that the light bulb has gone on and I realized DMF is the answer to that other precursor, I agree that it is the better way
to go. Thanks for the comment.
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
First of all, thanks for all the interesting discussion.
Second, does anyone have thoughts on removing the bromine of
bromocriptine to leave you with ergocryptine?
I've read that this would entail making the Grignard of bromocriptine, then hydrolyzing it off leaving a hydrogen in its place. Would doing this
likely case side reactions?
I don't think this warrants a new thread as its so close to the starting subject, so I asked here.
[Edited on 26-12-2008 by BobHawson]
BobHawson
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
| Quote: |
See the Merck Index for bromolysergide. That is bromo-LSD. Inhibits serotonin but devoid of hallucinatory effects.
My guess is that a bulky Br vicinal to the indole N lone pair blocks interaction with receptor sites for those effects.
[Edited on 26-12-2008 by Sauron] |
Which receptor sites do you mean? It seems to affect serotonin receptors.
BOL-148. 2-Bromo-N,N-diethyllysergamide.
At 6 to 10 milligrams orally, there are some mental changes noted. But in another study, 20 milligrams was administered a day to a subject for 7 days,
and there were no reported effects. And yet it is as potent a serotonin agonist as is LSD.
taken from http://www.erowid.org/library/books_online/tihkal/tihkal26.s...
Apologies if posting from books is against forum rules.
[Edited on 26-12-2008 by BobHawson]
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Posting from books is not at all prohibited but it is customary to cite sources.
I simply meant, whatever receptor sites are involved in the hallucinatory effects, which the 2-bromo derivative does not posess. But the parent
compound does. I speculated that a bulky substituent vicinal to the N at 1 might sterically hinder interaction. But this was merely idle speculation,
I know nothing of the neuropharmacology of these substances.
Is 2-bromo-PA commercially available? Another member wanted to know.
See upthread re:Grignard and hydrolysis and possible side reactions. Posting the structure of bromocristine would be helpful.
Ergocristine is another ergot alkalois fragment that has been exploited clandestinely as a precursor to LA and LSD. The others that come to mind are
ergotamine, elymoclavine, ergotinine, and paspalic acid. Are we going to discuss them one by one in terms of the 2-bromo derivs, or should we cover
them as a class?
Oh, and that list is far from exclusive.
-----------------------
I tracked down that indole prep talked about in Synlett 1994, they referenced a paper (ref.6) from Chem.Pharm.Bull. which is open access. The SynLett
procedure was modified from the Japanese one, and I needed to go two papers back in CPB to volume 29 to find the details.
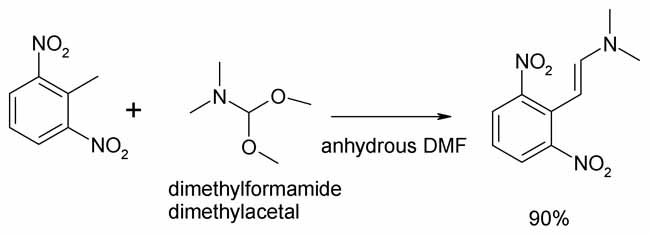
The Japanese authors prepared 4-nitroindole and 4-aminoindole by the titanium(III) chloride reduction (in aqueous AcOH) of the enamine formed from
2,6-dinitrotoluene and dimethylformamide dimethylacetal. Thus 2,6-dinitro-N,N,-dimethyl-trans-beta-styrene is cyclized to the 4-substituted indoles.
Anhydrous DMF is solvent for the prep of the enamine, 90% yield.
The Synlett authors substituted (apparently) 2-bromo-6-nitrotoluene in the enamine prep, and used Fe powder in AcOH rather than TiCl3 as reducing
agent.
Dimethylformamide dimethylacetal is commercially available.
So this route just got easier. The requisite nitrobromotoluene will be easy to prepare from o-bromotoluene nitration or bromination of o-nitrotoluene.
Doubtless there is lit. on this.
The Japanese authors also got to the 4-halo indoles via diazotization of 4-aminoindole.
I will assemble the CPB articles into a single pdf and post here.
[Edited on 27-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
Next time I'll mention what book it was from, but I did give a link to the book.
2-bromo-PA is not commercially available, however 2 Bromo alpha ergocryptine (bromocriptine) is.
I posted a hyperlink to the structure of bromocriptine, and asked how to replace the Br with an H to get ergocryptine (which I also gave a hyperlink
for).
I'll post the actual pictures though.
Bromocriptine

Ergocryptine

We ought to cover them as a class, I think, unless there are significant differences.
And ergocristine. I assume bromocristine would have a bromine in the same postion as bromocriptine

Sorry if its all a bit confusing!
[Edited on 26-12-2008 by BobHawson]
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Assuming that the Br are all at the indole C-2 then your problem remains very much the same as with PA. Find a base or reducing agent that will
replace Br with H and not affect anywhere else on the structure. Given the diversity it may not be same reagent for all.
Some experimentation may be called for.
--------------
The Japanese papers are rather large so I will post them one by one in order of publication.
[Edited on 27-12-2008 by Sauron]
Attachment: 29_726.pdf (1.4MB)
This file has been downloaded 539 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The Chem Pharm Bull paper last post centers around use of this enamine for prep of 4-substituted indoles.
The authors used TiCl3 in wet AcOH to close the indole ring. They also used Zn/Hg amalgam as an alternative.
In subsequent work (SynLett 1994 posted above) others employed the same enamine reagent with 2-bromo-6-nitrotoluene and also substited Fe powder, AcOH
and silica gel for the reduction/cyclization.
[Edited on 27-12-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
What bases or reducing agents do you all think would be applicable?
NaOH or KOH seem to be likely candidates. Would they likely work well?
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
| Quote: | Originally posted by BobHawson
What bases or reducing agents do you all think would be applicable?
NaOH or KOH seem to be likely candidates. Would they likely work well? |
Not in cases like paspalic acid. They may remove the Br but will also isomerize the structure to lysergic acid with a large (too large) amount of
isolysergic acid as well.
According to the patent literature, a quaternary ammonium base (PTC) is used to get the isomerization without the epimerization. Will this reagent
also take off your Br? I do not know. The answer is in the lit. somewhere, and if not, you need to experiment. I don't think anyone on this forum has
this answer in his head, nor are they likely to want to spoonfeed you even if they do. But, I could be wrong.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The intermediate followup paper from Chem Pharm Bull vol 29
[Edited on 27-12-2008 by Sauron]
Attachment: jnlpdf2.pdf (1.4MB)
This file has been downloaded 563 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The third and final of the CPB Japan papers on prep of 4-substituted indoles
This also covers building of the 3-acetonitrile side chain on such indoles as requisite for the unrelated work in SynLett 1994.
BTW this reaction is called
The Leimgruber-Batcho Indole Synthesis
There's an Org.Syn. monograph by Botcho and Leimgruber on this reaction.
I found a JOC paper on it as well, using the Fe powder/AcOH reducing system as in SynLett
And the Org.Syn. paper references a Ber. preparation for the DMF-dimethylacetal. Wiley's server is down but as soon as they get their act together I
will get the DOI for this and request it in References.
[Edited on 28-12-2008 by Sauron]
Attachment: jnlpdf3.pdf (1.4MB)
This file has been downloaded 540 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
Thank you for the literature, will make for interesting reading.
What about in the cases of bromocriptine and bromocristine?
NaOH or KOH would take off the bromine, but I assume they would also hydrolyze off half the molecule in the same reaction. It seems they would leave a
mix of LA and predominately iso-LA.
Now how would one just remove the Br without hydrolyzing off the other portion of the molecule?
BobHawson
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
I haven't, could you post it or give a link to it?
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I have dug up four last papers to tidy up loose ends re prep of 4-bromoindole.
Here are the first two:
-- a review of the Leimgruber-Batcho indole synthesis
-- Broderick's prep of DMF-DMA.
Enjoy!
[Edited on 28-12-2008 by Sauron]
Attachment: ergot.alkaloids.chem.rev.pdf (2.6MB)
This file has been downloaded 761 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
BobHawson
Harmless

Posts: 34
Registered: 10-12-2008
Location: USA
Member Is Offline
Mood: Regular
|
|
I assume you mean Stoll?
BobHawson
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Good assumption.
Here are the last 2 papers dealing with the prep of 4-bromoindoles:
-- Org Syn prep by Batcho and Leimgruber for whom rxn is named
-- a JOC note describing the use of Fe/AcOH as the reducing system for cyclization in this reaction
I compiled these into a rar for convenience
[Edited on 28-12-2008 by Sauron]
Attachment: refs2.rar (317kB)
This file has been downloaded 566 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The enaminating agent in the Leimgruber-Batcho indole synthesis is, as already discussed, DMF-DEA and I posted Broderick's prep of this which employs
dimethyl sulfate.
Subsequently I found my copy of Paquette's book and extracted the section on this type of reagent, which includes two additional preparations, one
using chloroform and the other, the Vilsmeier reagent. Yields are not as high as reported by Broderick, not no nasty old dimethyl sulfate involved,
and for many of us that is a good tradeoff.
Also this paper describes many of the other reactions of this versatile and powerful orthoamide.
[Edited on 29-12-2008 by Sauron]
Attachment: DMFDMA.pdf (130kB)
This file has been downloaded 17103 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
As I recall, producing iso-lysergic acid isn't a terminal problem. The isomerization is reversible. Iso-lysergic acid may be isomerized back into
lysergic acid without too much fuss.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
That's true because LA and iso-LA are epimers.
That happy situation is not the case for D-LA vs L-LA. Which is to say (+)-LA vs (-)-LA. The Wang-Hendrickson produces racemic LA,
an equal mixture.
However as I am not going to make LA or any of its derivatives, this is not my problem.
[Edited on 8-1-2009 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
| Pages:
1
2 |