omario4
Harmless

Posts: 16
Registered: 25-8-2008
Member Is Offline
Mood: No Mood
|
|
Why isn't this structure valid?
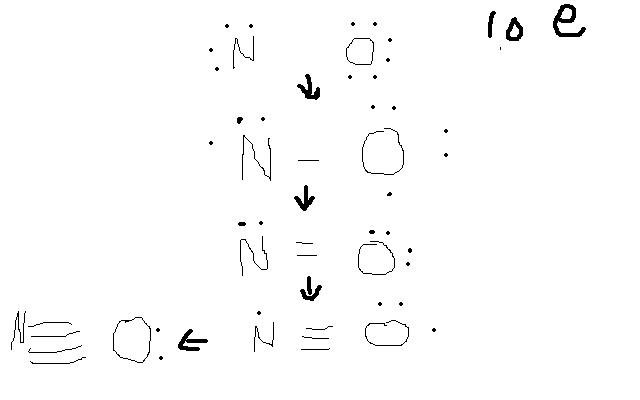
Using methods i was taught in class, the lewis structure for NO+
ion is a triple bond between the nitrogen and oxygen with each containing a lone pair.
I counted all the valence electrons, subtracted 1 and distributed them around oxygen to give it an octet and gave the remainder to the nitrogen.
However if i were to juts starts off with each having their valence electrons N 5 O 6 and take 1 off nitrogen to make the cation, then i pair off each
of nitrogen and oxygens electrons together to give them an octet, i'm left with a quadruple bond and lone pair on oxygen.
But the valid structure that i got from the other method seems to imply that oxygen is using up its own valence electrons more than nitrogens. I
uploaded the paint picture of what i did for alternate method. So if covalent bonding is the sharing of electrons shouldn't each bond result in both
molecules putting up an electron?
[Edited on 25-11-2008 by omario4]
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Nitric oxide is considered to be a free radical. It violates the octet rule with *11* electrons (O=6, N=5).
Check your periodic table, and see a synopsis:
http://userpages.umbc.edu/~budzicho/Chp11note2.html
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
omario4
Harmless

Posts: 16
Registered: 25-8-2008
Member Is Offline
Mood: No Mood
|
|
Ok, what i was having confusions with was how molecules go about making the bonds in the first place. do they have to pair each of their electrons one
from each atom at a time to make the first covalent bond etc? or are they more flexible than that.
Ok i see why my lewis structure was wrong, oxygen had more than octet.
[Edited on 25-11-2008 by omario4]
|
|
|
superman1451
Harmless

Posts: 6
Registered: 7-11-2007
Member Is Offline
Mood: No Mood
|
|
Can there even be a quadruple bond?
|
|
|
omario4
Harmless

Posts: 16
Registered: 25-8-2008
Member Is Offline
Mood: No Mood
|
|
Ya, a quadruple bond would have too much electron-electron repulsions. So basically the octet rule can be violated when there are insuffiicient or odd
numbers of electrons or if it involves elements in period 3 or higher. But for period 2 you can never exceed an octet due to there being only 8
orbitals in principle shell n = 2.
Thank you so much for your patience and help. I have to go to sleep soon i'll check back tomorrow.
|
|
|