
Amoled - 2-10-2018 at 12:37
Hey, would it be possible, to turn sulfonic acid groups, like in Taurine
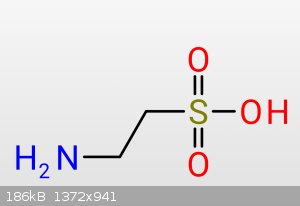
into "Thioamines" or how you would call it - like this
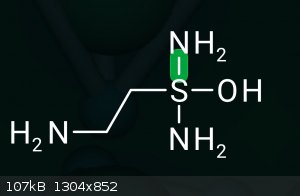
via Leuckart-Wallach for example?
And if not, is there any specific reason?
fusso - 2-10-2018 at 12:59
Why is S 4-valent in your molecule?
clearly_not_atara - 2-10-2018 at 13:00
Compounds with two geminal YHx substituents where Y = (N, O, Cl, F, S, Br, I) x = (0,1,2) tend to be very unstable, eliminating YH(x+1). So your
example compound would eliminate ammonia. However, the general class of compounds does exist:
https://www.ncbi.nlm.nih.gov/m/pubmed/14871110/
Edit: better link
[Edited on 2-10-2018 by clearly_not_atara]
Sigmatropic - 2-10-2018 at 13:08
Sulfonamide of taurine is probably known. what you have drawn is some vastly more obscure compound which would be best described by replacing the HO-S
with a S=O. These are apparently called sulfonimidamides and are not prepared by reductive amination of some S=O compound and neither are
sulfonamides.
https://onlinelibrary.wiley.com/doi/10.1002/adsc.201800273
https://www.organic-chemistry.org/Highlights/2004/24December...
