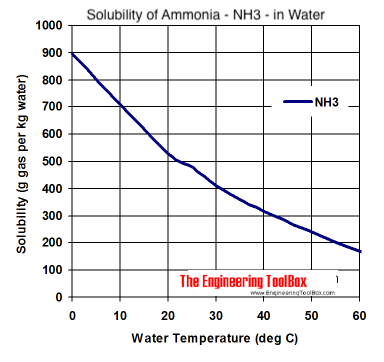
I haven't been able to find a solubility chart for ammonia at higher temperatures, but I did find a quote from another sciencemadness thread | Quote: | | Drive out ammonia using heat (not boiling), and redissolve in small amount of cold water. Problem is that at 100C water still holds 7% ammonia, which
is more than household ammonia (but janitorial ammonia is 10%). |
http://www.sciencemadness.org/talk/viewthread.php?tid=23666&...
No source was given for this, but if it's true, it would explain why the solution boiled before all of the ammonia had been driven off. I don't know
if it technically counts as an azeotrope, but it would definitely have a similar effect on distillation/reabsorption of ammonia. Other than filtering
the calcium sulfate out, I suppose you could just distill the whole reaction mixture until most of the water (and ammonia too) has came over. Of
course, that would take a lot of time and energy. Another thing to do would be to use less water. It doesn't have to be enough to dissolve all the
ammonium sulfate at once, I wouldn't think. If even a bit of it dissolves, it will react, creating (mostly) insoluble calcium sulfate, ammonia, and a
bit more water. Of course, now you have to figure out a way to capture all the ammonia quickly, since it will now outgas as the reaction precedes.
Maybe you could put a beaker full of water upside down in a bigger container of ice water, then put the bubbling tube underneath it to trap the
ammonia until it can dissolve. I suppose there are ways to capture NH3 without water, but the solutions I thought of (gas bag, compressed cylinder,
condensing using low temperature or very high pressure) would be hard to do safely in a home lab. |









 I do have some weak cleaning ammonia. If I get a chance to try boiling gas off of it,
I'll post the results. Worst that could happen (barring some accident), I would have some non-soapy ammonia solution.
I do have some weak cleaning ammonia. If I get a chance to try boiling gas off of it,
I'll post the results. Worst that could happen (barring some accident), I would have some non-soapy ammonia solution.