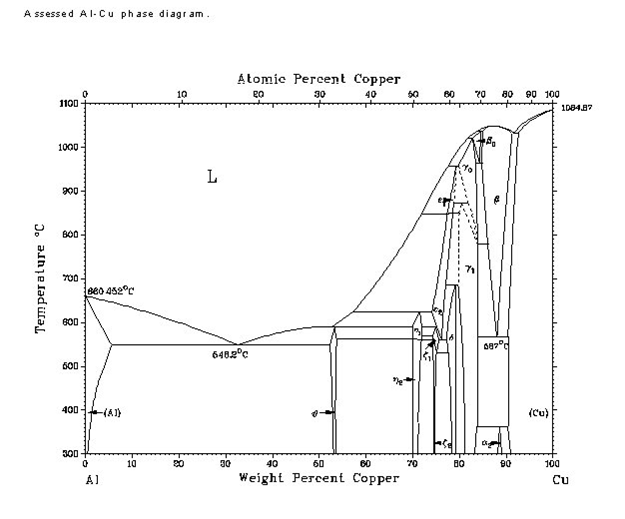
IMITATION GOLD.
I. One hundred parts, by weight, of copper of the purest quality; 14 of zinc or tin; 6 of magnesia; 3/6 of sal ammoniac, limestone, and cream of
tartar. The copper is first melted, then the magnesia, sal ammoniac, limestone, and cream of tartar in powder are added separately and gradually. The
whole mass is kept stirred for a half hour, the zinc or tin being dropped in piece by piece, the stirring being kept up till they melt. Finally the
crucible is covered and the mass is kept in fusion 35 minutes and, the same being removed, the metal is poured into molds, and is then ready for use.
The alloy thus made is said to be fine-grained, malleable, takes a high polish, and does not easily oxidize.
II. An invention, patented in Germany, covers a metallic alloy, to take the place of gold, which, even if exposed for some time to
the action of ammoniacal and acid vapors, does not oxidize or lose its gold color. It can be rolled and worked Tike gold and has the appearance of
genuine gold without containing the slightest admixture of that metal. The alloy consists of copper and antimony in the approximate ratio of 100 to 6,
and is produced by adding to molten copper, as soon as it has reached a certain degree of heat, the said percentage of antimony.
When the antimony has likewise melted and entered into intimate union with the copper, some charcoal ashes, magnesium, and lime spar are added to the
mass when the latter is still in the crucible.
III. Aluminum Gold. This alloy, called Nuremberg gold, is used for making cheap gold ware, and is excellent for this purpose, as its
color is exactly that of pure gold, and does not change in the air.
Articles made of Nuremberg gold need no gilding, and retain their color under the hardest usage; even the fracture of this alloy shows the pure gold
color. The composition is usually 90 parts of copper, 2.5 of gold, and 7.5 of aluminum.
IV. Imitation gold, capable of being worked and drawn into wire, consists of 950 parts copper, 45 aluminum, and 2 to 5 of silver.
V. Chrysochalk is similar in composition to Mannheim gold:
I II
Copper 90.5 58.68
Zinc 7.9 40.22
Lead 1.6 1.90
In color it resembles gold, but quickly loses its beauty if exposed to the air, on account of the oxidation of the copper. It can, however, be kept
bright for a long time by a coating of colorless varnish, which excludes the air and prevents oxidation. Chrysochalk is used for most of the ordinary
imitations of gold. Cheap watch chains and jewelry are manufactured from it, and it is widely used by the manufacturers of imitation bronze ornaments.
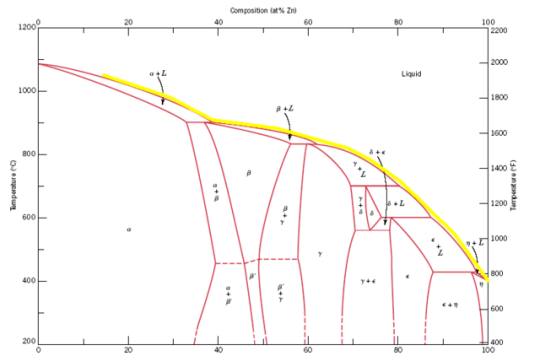
Mannheim Gold or Similor. Mannheim gold is composed of copper, zinc, and tin, in proportions about as follows:
I II
Copper 83.7 89.8
Zinc 9.3 9.9
Tin 7.0 0.6
It has a fine yellow color, and was formerly much used in making buttons and pressed articles resembling gold. Later alloys, however, surpass it in
color, and it has fallen somewhat into disuse.
One variety of Mannheim gold, so called, contains 1.40 parts of brass (composition 3 Cu 2 1 Zn) to 10 of copper and 0.1 of zinc.
Mosaic Gold. This is an alloy composed with slight deviations of 100 parts of copper and 50 to 55 of zinc. It has a beautiful color,
closely resembling that of gold, and is distinguished by a very fine grain, which makes it especially suitable for the manufacture of castings which
are afterwards to be gilded. The best method of obtaining a thoroughly homogeneous mixture of the two metals is first to put into the crucible
one-half of the zinc to be used, place the cover upon it, and fuse the mixture under a cover of borax at as low a temperature as possible. Have ready
the other half of the zinc, cut into small pieces and heated almost to melting, and when the contents of the crucible are liquid throw it in, a small
portion at a time, stirring constantly to effect as intimate a mixture of the metals as possible.
Oreiide or Oroide (French Gold). The so-called French gold, when polished, so closely resembles genuine gold in color that it can
scarcely be distinguished from it. Besides its beautiful color, it has the valuable properties of being very ductile and tenacious, so that it can
easily be stamped into any desired shape; it also takes a high polish. It is frequently used for the manufacture of spoons, forks, etc., but is
unsuitable for this purpose on account of the large amount of copper contained in it, rendering it injurious to health. The directions for preparing
this alloy vary greatly. The products of some Paris factories show the following composition:
I II III
Copper 90 80.5 86.21
Zinc 10 14.5 31.52
Tin 0.48
Iron 0.24
A special receipt for oreïde is the following:
IV. Melt 100 parts of copper and add, with constant stirring, 6 parts of magnesia, 3.6 of sal ammoniac, 1.8 of lime, and 9 of crude tartar. Stir
again thoroughly, and add 17 parts of granulated zinc, and after mixing it with the copper by vigorous stirring keep the alloy liquid for one hour.
Then carefully remove the scum and pour off the alloy.
Pinchbeck. This was first manufactured in England. Its dark gold color is the best imitation of gold alloyed with copper. Being very
ductile, it can easily be rolled out into thin plates, which can be given any desired shape by stamping. It does not readily oxidize, and thus
fulfills all the requirements for making cheap jewelry, which is its principal use.
Copper 88.8 93.6
Zinc 11.2 6.4
|



 I didn't realize Pinchbeck was made to resemble 18K; that's good to know.
I didn't realize Pinchbeck was made to resemble 18K; that's good to know.













 https://www.google.com/search?q=handheld+xrf&safe=active...
https://www.google.com/search?q=handheld+xrf&safe=active...