
QuestionableEthics - 8-12-2017 at 20:57
Hi.
I'm reading a workup that originated on The Hive (http://chemistry.mdma.ch/hiveboard/novel/000058487.html#Post...) and is archived now on Rhodium/Erowid (https://erowid.org/archive/rhodium/chemistry/methylenation.h...).
It's for the demethylation of eugenol, which has been discussed elsewhere on the board. However, rather than necropost I thought I'd ask a few very
specific questions, and see if anyone can offer any insight.
1. The workup uses a complicated filtration procedure at the end. I'm wondering why this is necessary. It seems to me that a benzenediol with an
aliphatic substitution should be fairly insoluble in water, and fairly soluble in the cyclohexane. So why can't simple separation be used, followed by
distilling off the cyclohexane? Is it something to do with the usage of the PTC, increasing the solubility of the Lewis acid in the organic layer? But
my understanding is that once the reaction mixture is cooled, this is no longer relevant.
2. In the patent EP2620424A1 (accessible here: https://patents.google.com/patent/EP2620424A1/en - relevant portion at line 35), the preference seems to be perform an oxidation of the allyl
substitution prior to demethylation. Is this because of a risk of a halogen addition across the double bond? Is there any work quantifying the
occurrence of this side reaction if so, maybe comparing different Lewis acids?
I realize these questions are pretty difficult to answer without experimentation; but my inability to answer them *at all* seems to suggest a lack of
some fundamental knowledge, so any guidance would be appreciated. I've lurked here for a long time, looking forward to hopefully being able to
contribute a bit. I realize cookery is pretty frowned upon here, for good reason, so I hope my questions don't cross that line. In case they do,
references to similar reactions/workups would be appreciated to gain an understanding of the concepts at play here.
Thanks for reading.
Sigmatropic - 9-12-2017 at 01:13
Your link has many reactions in it. I believe you mean this one but I'm just guessing.
TextReaction with Eugenol
In a dropping funnel, eugenol (1 mol), tetrabutylammonium iodide (1/360 mol) and a little cyclohexane are combined. This is slowly dripped into the
AlI3/cyclohexane mixture made in the previous step with vigourous stirring and inert gas sheilding. After all the eugenol has been added the solution
is brought to reflux (it is a tan coloured slurry at this point, but upon refluxing turns less viscous) for 1 hour. The solution is then cooled in an
ice bath and hydrolysed with ~600mL of water, slowly.
The resultant mix is filtered under nitrogen and the solid collected immersed in ethyl acetate, this is swirled for a couple of minutes, then
filtered. The ethyl acetate is seperated from the water (collected through the filtration) and removed and what you have left is a tan coloured solid
with a charactistic 'smoke' smell and a melting point 45-51°C, althought this range could be tightened by recrystalisation or vac distillation with
hot water in the condenser.
A few notes:
If you are quick then maybe you can not bother with the inert atmoshpere in the workup, however the filtering takes a long time (don't even consider
using gravity) beacause the Al is very finely divided and clogs up the filter paper. The use of a filtering aid like Celite is recommended.
It seems the reactant ratios are critical so get them right.
The workup is a shit for large quantities, any suggested impovements would be appreciated.
Now then. First I would point out it is poorly written. Second they filter off insoluble aluminum salts which were formed because the work-up was
executed poorly. Look up fieser work-up and do some reading on Rochelle's salt.
Bert - 9-12-2017 at 08:37
One of my teachers used to say that free information on explosives you found on the internet is often worth exactly what you paid for it.
I would guess online information for this branch of organic chemistry to be similar.
Demethylation of eugenol, a better & more recent mechanism
QuestionableEthics - 14-12-2017 at 16:33
Sigmatropic, I agree, it seems pretty poorly written. Given the context in the forum, I can see why. And thank you for the references to those
keywords, I'll be sure to read up.
Bert, I agree with you as well. Were I to perform this reaction, I would most likely turn to the procedure worked out by Sang et al earlier this year.
I don't think I can attach the article due to size, but the DOI is 10.1055/s-0035-1588889 and using that one can pull it from Scihub if they're
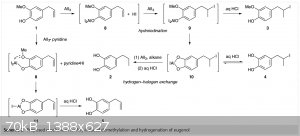
curious. I did attach a screenshot of the proposed mechanism.
[Edited on 15-12-2017 by QuestionableEthics]
Sigmatropic - 15-12-2017 at 10:07
I think articles can be attached, this would make discussion alot easier as people like me wouldn't run into paywalls.
Without having read the experimental and exact conditions, i.e. based on paper chemistry, I think the reaction of 1 with AlI3 and thus 8 and HI is
really an equilibrium between 1-iodo-1-(4'-cathechol)-propane (not drawn) and 2-iodo-1-(4'-cathechol)-propane) (compound 9). Reduction of said
benzylic iodides by HI is well known. Would be interesting to know what they comment on the formation of compound 3, or how they observed it, as that
is a pivital point for deciding on hydrogen-halogen exchange of 9 or the same reaction on the benzylic iodide.
I'm not very much suprised by the fact that control of pH (probably a misnomer as in organic solvents the concentration of H3O+ is negligible) affects
the outcome of a reaction and/or avoids the formation of side product. Original procedures usually employ the straight reagent (e.g. the knoevenagel
condensation is orignially ran neat in piperidine), Newer procedures, which have hopefully been optimized, runn in slightly more 'acidic' but still
netto basic mixture of piperperidine and the corresponding carbamate(1). I have ran such a condensation using a mixture of piperidine and piperidine
acetate (missing refernce). Controlling the 'pH' of this AlI3 reagent for demethylation of phenols seems to be more of the same but the other way
around; knoevenagel: make the base catalyst less basic vs. demethylation with AlI3: make the lewis acid catalyst less acidic.
1: http://www.organic-chemistry.org/abstracts/lit4/003.shtm
[Edited on 15-12-2017 by Sigmatropic]
Article
QuestionableEthics - 15-12-2017 at 23:00
Whoops, you're right, it is indeed less than 8MB. Attached it, yanked straight from Sci-Hub.
With regard to the possibility of an equilibrium for compound (9), I think this is a simple matter of carbocation stability right? The halide will
prefer the more highly substituted carbon. Of course I think the terminal iodo compound will also be formed in small amounts. I think (3) was observed
through reducing the ratio of the Lewis acid to the substrate.
I agree, this seems to be a natural progression for the synthesis procedure. Particularly because pyridine*HCl was used in the past with success
(second attached article). Unfortunately this one doesn't go into detail about the side reactions. I'd be curious to know where the 20% jump in yield
came from. Logic says it's the lack of an ortho hydroxyl group based on the mechanism, because the 1999 article didn't examine such substrates. In the
case of eugenol, I wonder how pyridine*HCl would compare to pyridine*AlI3 in the lab, yield-wise.
Attachment: tian2016.pdf (196kB)
This file has been downloaded 460 times
Attachment: kulkarni1999.pdf (88kB)
This file has been downloaded 310 times
