
Σldritch - 5-9-2017 at 11:23
I thought it was polymerization products since nitromethane gets more orange as it is stored but the color carries over with distillation. Maybe the
short chains carries over?
Also there is supposed to be some dark - very explosive - oil left over from http://www.sciencemadness.org/talk/viewthread.php?tid=5026&page=3 Nitromethane destillations, presumably that is what gives it color but that
raises the question why some of it carries over.
hissingnoise - 5-9-2017 at 12:15
Even miniscule amounts of NO2, a decomposition product, colour NM yellow/orange and since NM is produced in the nitrolysis of propane, some
impurities are to be expected...
alking - 6-9-2017 at 07:36
If it's NO2 you should be able to boil it off and the NO2 will outgas. Of course I wouldn't bother unless NO2 will harm your reaction.
Σldritch - 6-9-2017 at 09:53
I can not come up with anything better than nitrogen dioxide but i still find it unlikely as an explanation. Ill try to make a somewhat coherent
explanation.
1. Yellow is a understatement. It is more orange, more orange than RFNA, which has a few percent Nitrogen Dioxide in it.
2. Which you would definetly smell, especially on boiling.
3. On fractionally distilling a slightly yellow, around azeotropic methanol-nitromethane solutiona the yellow/orange stuff concentrates.
4. On freezing you would expect the color to disapear if it is nitrogen dioxide because it would form colorless dinitrogen tetroxide, but it does
not...
alking - 6-9-2017 at 11:10
1.) It doesn't rule it out, pure liquid NO2 is probably red or some rather dark shade of orange -> brown. Even baring a higher percentage in the
nitromethane it could have a darker appearance based on solubilities/light diffraction, etc.
2.) I would expect so, yes.
3.) How does it come over when you fractionate it? Does it come over first, last, or can you not really separate it easily? I didn't quite comprehend
what you were saying here.
4.) What do you define as freezing though? The colder you go the more the equilibrium shifts towards N2O4 but at 0C it's far from fully shifted to the
right, you'll still have a substantial amount of NO2 and thus a non colorless solution.
Melgar - 6-9-2017 at 21:22
Nitromethane will form a red-colored product in the presence of a strong base catalyst. Could that be it?
woelen - 6-9-2017 at 22:40
Nitromethane forms very dark red compounds in contact with alkali. Just try adding a drop of nitromethane to a dilute solution of NaOH in water. It
will dissolve slowly and the liquid turns red. In the later stages of the experiment, the liquid will become very dark.
Many types of glass leach out small amounts of alkali, enough to give a a clearly visible color to nitromethane.
The nature of these red compounds is not known to me. I think that it is some condensed species, not a single entity, but material with a general
structure. Many organics do this kind of things under the right circumstances (another example is acetone, to which strong acid, such as a drop of
H2SO4, is added).
Σldritch - 7-9-2017 at 07:00
(Speculation warning)
Nitromethane might be able to undergo something similar to the nitroaldol reaction or aldol condensation giving products of varying molecular length.
The shorter ones are distilled with the nitromethane and gives it yellow color while the longer ones remains and give the red color.
If someone feels brave and/or has a lot of nitromethane you might be able to concentrate some of the lower polymerization products by soxhlet
extracting some base insouble in nitromethane. See this diacetone alcohol synthesis: http://orgsyn.org/demo.aspx?prep=cv1p0199
I dont think you would be able to use any hydroxides because of these reactions: http://www.sciencemadness.org/talk/viewthread.php?tid=1089
Maybe calcium methoxide with azeotropic methanol/nitromethane would work. This sounds way too dangerous for me though.
How about this mechanism?
[Edited on 7-9-2017 by Σldritch]
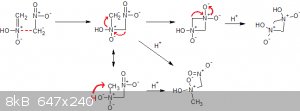
zed - 10-9-2017 at 15:54
Now for an alternate view: Nitromethane can become sensitized aka unstable. You don't want that. Unstable means explosive.
Apparently, some racing fuel now has an indicator in it, to warn you of instability.
http://nitromater.com/threads/color-of-nitro-methane.34861/
alking - 11-9-2017 at 05:15
How real is the risk of that occurring, and how would one either treat it after, stabilize it before, or test for it if unknown?
